ABSTRACT
Purpose: To evaluate membranes originating from pure or oxidized bacterial cellulose (BC)/alginate/strontium apatite hydrogels regarding toxicity, biocompatibility, biodegradation and metabolism.
Methods: The toxicity was measured by incubating the materials with Artemia salina for 24 h, and mortality and the 50% lethal concentration were determined in comparison to potassium dichromate by Probit analysis. Local biocompatibility and biodegradation were evaluated by subcutaneous assay in 75 Swiss mice; the test groups were compared to sham and collagen membrane at one, three and nine weeks. The histopathology of tissue irritation followed the ISO 10993-6 standard, and the integrity of the biomaterials scored by quartiles. Metabolic analysis of relative weight and the intensity of catalase, iodine and nitrite were carried out for liver, kidneys and tibias of the tested animals.
Results: All cellulose-based materials were nontoxic, biocompatible, and none presented nitrosative stress. The oxidized BC was more resorbable, and the non-oxidized BC had greater renal biochemical reactivity.
Conclusion: The membranes suggest applicability as regenerative barriers. However, long-term studies in bone defects are necessary to elucidate their osteopromoting efficiency.
Key words
Cellulose; Strontium; Hydroxyapatites; Materials Testing; Biocompatible Materials; Guided Tissue Regeneration

 Evaluation of toxicity, local biocompatibility, biodegradation, and systemic metabolism of cellulose/alginate/strontium apatite membranes implanted subcutaneously in mice
Evaluation of toxicity, local biocompatibility, biodegradation, and systemic metabolism of cellulose/alginate/strontium apatite membranes implanted subcutaneously in mice Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Source: Elaborated by the authors.
Source: Elaborated by the authors.
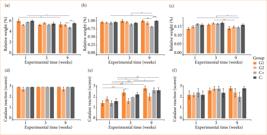
 *p = 0.01–0.04, **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
*p = 0.01–0.04, **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
 *Implanted material; white arrow: mixed inflammatory infiltrate; black arrow: giant cell; arrowhead: blood vessel; circle: fibrosis. Staining: hematoxylin-eosin. Magnification: 1,600 x. Scale bar: 600 μm. Source: Elaborated by the authors.
*Implanted material; white arrow: mixed inflammatory infiltrate; black arrow: giant cell; arrowhead: blood vessel; circle: fibrosis. Staining: hematoxylin-eosin. Magnification: 1,600 x. Scale bar: 600 μm. Source: Elaborated by the authors.
 *p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
*p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
 *p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
*p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
 *p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
*p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
 *p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.
*p = 0.01–0.04; **p = 0.001–0.009; ***p < 0.001. Source: Elaborated by the authors.