Abstract
Background Pistia stratiotes L., is an invasive aquatic plant that forms dense mats, disrupting ecosystems and affecting waterway navigation. Although various control methods have been studied, there is no clear consensus on the best approach.
Objective The goal of this study is to evaluate the efficacy of various control methods in managing P. stratiotes infestations.
Methods Laboratory trials tested the efficacy of diquat, d-limonene, mineral oil, and potassium salts of fatty acids on P. stratiotes. Treatments were applied at concentrations from 10–30%, with weekly monitoring for five weeks to assess treated plants visual quality (injury symptoms), and dry biomass. Greenhouse trials replicated the most effective laboratory treatments.
Results In laboratory trials, diquat at 0.89% achieved > 90% reduction in visual quality and dry biomass of P. stratiotes, making it the most effective treatment. D-limonene at 30% showed similar efficacy, while mineral oil also significantly reduced P. stratiotes populations. Combinations of d-limonene with mineral oil or potassium salts of fatty acids exhibited synergistic effects; some combinations achieved >90% reduction in visual quality and dry biomass. Treatments prepared with seawater with diquat (0.89%) and d-limonene (20% and 30%) were the most effective treatments. Greenhouse trials confirmed these results, suggesting these treatments could be effective.
Conclusions In conclusion, this study underscores the effectiveness of diverse strategies in controlling P. stratiotes infestations. Further research is warranted to evaluate long-term efficacy, cost-effectiveness, and environmental impact. Integrated approaches offer a multifaceted and sustainable solution for managing P. stratiotes, mitigating its ecological and economic impacts.
Invasive Species Control; Aquatic Plant Management; Herbicidal Treatments; Chemical Control Methods; Plant Population Reduction; Ecosystem Restoration; Integrated Weed Management; Environmental Impact Assessment
1. Introduction
Pistia stratiotes L., commonly known as water lettuce, originates from the Americas and has spread globally due to human activities such as the aquarium trade and accidental dispersal (European and Mediterranean Plant Protection Organization, Organisation Europénne et Méditerranéenne pour la Protection des Plantes, 2017; Hussner, 2014). Its rapid proliferation and buoyant nature have enabled its colonization in various water bodies worldwide. Initially recorded in the EPPO (European and Mediterranean Plant Protection Organization) region in the Netherlands in 1973 (Mennema, 1977), it has since become established in multiple EPPO zones and is regulated as an introduced species (European and Mediterranean Plant Protection Organization, Organisation Europénne et Méditerranéenne pour la Protection des Plantes, 2017). In Morocco, it was first observed in 2012 (Hill, 2013), leading to its classification as a quarantined organism by the Ministry of Agriculture.
Managing the growth and spread of P. stratiotes is essential for mitigating its negative impacts on aquatic ecosystems. The overgrowth of floating vegetation poses several challenges, including reduced oxygen and light penetration into the water, creation of monocultures by outcompeting native plants, and interference with flood control operations through the formation of dense mats that obstruct canals and water flow structures (Gettys, Moore, 2019).
The management of P. stratiotes in aquatic ecosystems involves a comprehensive approach incorporating chemical, mechanical, physical, and biological methods. Aquatic herbicides, regulated by the U.S. Environmental Protection Agency (USEPA) to minimize environmental impact, are cautiously employed, taking into account economic and social factors (USEPA, 1996). However, public concerns about herbicide use have led to temporary suspensions of chemical weed management activities by organizations like the Florida Fish and Wildlife Conservation Commission (FWC) (2019). Stakeholder feedback emphasizes the need to reduce chemical usage in favor of environmentally friendly alternatives. Mechanical harvesting and physical removal are available options but are often costlier and less effective than chemical methods (Queensland Government, 2017). The persistence of P. stratiotes, with its ability to reproduce vegetatively and from seeds, requires ongoing management efforts over several years (Millane, Caffrey, 2014). Chemical control, utilizing herbicides such as glyphosate and diquat, can achieve significant biomass reduction (Martins et al., 2002; Emerine, 2010), but eradication of large populations necessitates repeated applications, with the risk of re-infestation from untreated plants and seed (Glomski, Mudge, 2013). Biological control options, involving phytophagous insects, show promise but face challenges due to the predominance of generalist species (Cordo, Sosa, 2000). Despite these challenges, significant financial resources are allocated to P. stratiotes management worldwide, reflecting its substantial ecological and economic impact (Center, 1994). In Florida, annual costs for controlling P. stratiotes exceed 2 million USD, with over 100,000 USD spent by other eastern US states annually (Center, 1994). Over the past four decades, the combined yearly expenditure for managing both P. stratiotes and Eichhornia crassipes (Mart.) in Florida amounts to approximately 4.5 million USD (Center, 1994).
Natural herbicides, favored by home gardeners, organic farmers, and others seeking alternatives to synthetic herbicides, encompass a range of products including acids (such as acetic acid or vinegar and citric acid), oils (like clove, pine, peppermint, and citronella), soaps, iron- or salt-based herbicides, corn gluten, and combinations thereof (Smith-Fiola, Gill, 2017). These substances disrupt cell membranes, leading to plant desiccation and death (Baker, 1970; Webber III et al., 2018). Among commercially available natural herbicides, acetic acid is commonly listed as the active ingredient, typically at a concentration of 20% (Bradfield Industries, 2019). However, not all acetic acid products are designated for weed control, with horticultural vinegar containing 30% acetic acid being available but not explicitly labeled as an herbicide (Bradfield Industries, 2019). While citric acid is less prevalent, some products contain d-limonene, a citrus oil, as the active component (Cutting Edge Formulations). The scientific literature regarding the efficacy of natural products like acetic acid and d-limonene as weed control agents remains limited, both alone and in combination. Research suggests that acetic acid at concentrations of 20% or 30% may offer a viable alternative to glyphosate, though multiple applications may be necessary for sustained weed control (Domenghini, 2020). Acetic acid acts as a contact herbicide, damaging only the parts of the plant it directly touches. In contrast, glyphosate is a systemic herbicide that is absorbed and transported throughout the entire plant, leading to complete plant death. Additionally, there is limited data on the effectiveness of natural products like acetic acid in aquatic ecosystems. While acetic acid and d-limonene have shown some efficacy in weed control in certain contexts (Shrestha et al., 2012; Quarles, 2010; Gettys et al., 2021; Gettys et al., 2022; Gettys et al., 2023), their impacts on aquatic fauna and ecological toxicity are not well understood (Stubbs, Layne, 2020). These products are non-selective and may cause damage to off-target flora and fauna (Anderson, 2007). Therefore, their application in aquatic environments warrants careful consideration and further investigation to evaluate potential environmental risks (Saha et al., 2006).
The primary objectives of this project included evaluating the effects of several natural products, such as acetic acid, d-limonene, mineral oil, and potassium salts of fatty acid, both individually and in combination, on P. stratiotes. Diquat dibromide, a nonselective herbicide, was the most commonly used herbicide for managing floating weeds, with a total application of >7,800 gallons of formulation (37.3% diquat dibromide) (Clark, Dew, 2019). Therefore, it was used as the positive control treatment.
2.Material and Methods
2.1 Plant Material and Growth Conditions
Pistia stratiotes L. plants were collected in 2023 from Barrage Koreima in the Rabat-Sale-Kenitra region (33°49’17”N 6°49’29”W), Morocco. The plants were harvested by manually removing them from the water, ensuring minimal damage to the roots and foliage. They were then transferred to plastic containers (60 cm in length, 45 cm in width, and 40 cm in height) (Goodluck products, China). The containers were filled with water and moved to the greenhouse at the Experiment Domain of the National Institute of Agricultural Research (INRA) located in the Zemamra locality (32° 37' N, 8° 42' W). The plants were acclimatized and cultured under semi-controlled environmental conditions, including a temperature range of 25–27 °C, and natural light. A soluble fertilizer with a composition of 3N-3P-2K (Timac Agro, France) was added to every 10 liters of water present in the containers. The water in the containers was changed every two weeks to maintain appropriate nutrient levels, and minimize the accumulation of undesirable substances. Twenty plants were placed in each container and were grown for 4 to 6 weeks to achieve more than 80% surface coverage. Subsequently, the young plants were transferred to approximately 100 plastic containers of the same dimensions and acclimated under similar conditions to complete their development and ensure sufficient vegetative material for the experiments.
2.2 Laboratory trials
The study investigated the impact of three natural products on P. stratiotes L under controlled laboratory conditions (25 ± 2 °C, 66 ± 5% RH, and a 12:12 h (L:D) photoperiod).The products evaluated were Limocide (60 g d-limonene/L) (Vivagro, Martillac, France), Insecticide 101 (780 g mineral oil/L) (UPL, Ankleshwar, India), and Hamper (500 g potassium salts of fatty acid/L) (Gowan Crop Protection, Faenza, Italy). Each product was applied individually at concentrations of 10%, 15%, 20%, and 30%. Furthermore, all treatments were also applied in combination with each other. These products were chosen based on their distinct modes of action (Gettys et al., 2021). As a positive control, 37.3% diquat dibromide (Tribune Herbicide; Syngenta Crop Protection, Greensboro, NC) at a concentration of 0.89% was included due to its known efficacy against floating weeds as indicated in the FWC National Pollution Discharge Elimination System report for the calendar year 2018 (Gettys et al., 2021). All treatments, including the controls, were prepared using both tap water and seawater. Thus, each treatment underwent two preparations: one using tap water (Trial 1) and the other using seawater (Trial 2) (Table 1). The experiments were conducted on 30 22-day-old P. stratiotes plants (seed dispersal stage), each measuring 32.8 cm in height and 17.8 cm in width, which was ideal for observing the effects and symptoms of the tested products. These plants were grown in plastic containers (40 cm length × 30 cm width × 30 cm height) filled with water containing a soluble fertilizer with a composition of 3N-3P-2K (Timac Agro, France) for every 10 liters of water. The water and fertilizer were replaced monthly to maintain optimal growing conditions. The treatments were applied as a mist over the above-water foliage at a rate of 30 mL per plant using a professional 2L manual PE garden pressure hand sprayer (Taizhou Huangyan Jiaji Mould Plastic Co.,Ltd., China) with a pressure of 2 bar to ensure complete coverage. Ten replicates (containers) were used for each tested treatment, utilizing a completely randomized experimental block design. All experiments were conducted twice.
Plants were monitored weekly for 5 weeks post-treatment, and a numerical scale ranging from 0 to 10 was used to describe visual quality, as reported by Gettys et al. (2021) (0 = dead; 5 = fair quality, acceptable, somewhat desirable form and color, little to no chlorosis or necrosis; 10 = excellent quality, perfect condition, healthy and robust, excellent color and form). While previous studies have reported visual injury caused by herbicide treatments (Cutelle et al., 2013; Koschnick et al., 2005; Mudge et al., 2007), our research focused on evaluating visual quality. This parameter (treated-plant visual quality) has been utilized in assessing plant responses to various conditions (e.g., Gettys, Moore, 2018; 2019), herbicides (e.g., Gettys, Haller; 2012; Smith et al., 2014), salt stress (e.g., Tootoonchi et al., 2020), and other experimental factors. Following visual scoring, a destructive harvest was conducted to collect live biomass by carefully removing the plants from the containers. The collected materials were placed in paper bags, dried in a forced-air oven at 65 °C for 2 weeks, and then weighed. Visual data underwent arcsine transformation to ensure a normalized distribution. The tested treatment means of visual values and dried biomass were compared with untreated controls (tap water) using one-way ANOVA at a significance level of P = 0.05. Tukey’s LSD test was utilized for post-hoc comparisons. Analysis was performed using IBM SPSS Statistics version 23.0 software. Additionally, as proposed by Haller and Gettys (2013), an efficacy benchmark of >90% reduction in visual values and dried biomass parameters of target weeds compared to the untreated control was used.
2.3 Greenhouse trial
Following the results obtained in laboratory trials, the three treatments that displayed the highest efficacy (combinations of d-limonene and mineral oil, combinations of d-limonene and potassium salts of fatty acid, and combinations of mineral oil and potassium salts of fatty acid at 30% prepared using sea water) were chosen for greenhouse trials at 25 to 27 °C under natural light. Thirty 22-day-old P. stratiotes plants were grown in plastic containers measuring 60 cm in length, 45 cm in width, and 40 cm in height within a greenhouse. Treatments were applied at a rate of 30 mL per plant as a spray to ensure complete coverage of the leaves. Control plants were sprayed with either seawater or tap water. The experimental design was a completely randomized block design with 10 replicates (containers) for each tested treatment, and the experiment was conducted twice. Plants were monitored weekly for 5 weeks using the same numerical scale, destructive measures, and statistical analysis as in laboratory trials.
3.Results and Discussion
3.1 Laboratory trials
3.1.1 Impact of applying d-limonene, mineral oil, and potassium salts of fatty acids (prepared with tap water) on the population density of Pistia stratiotes
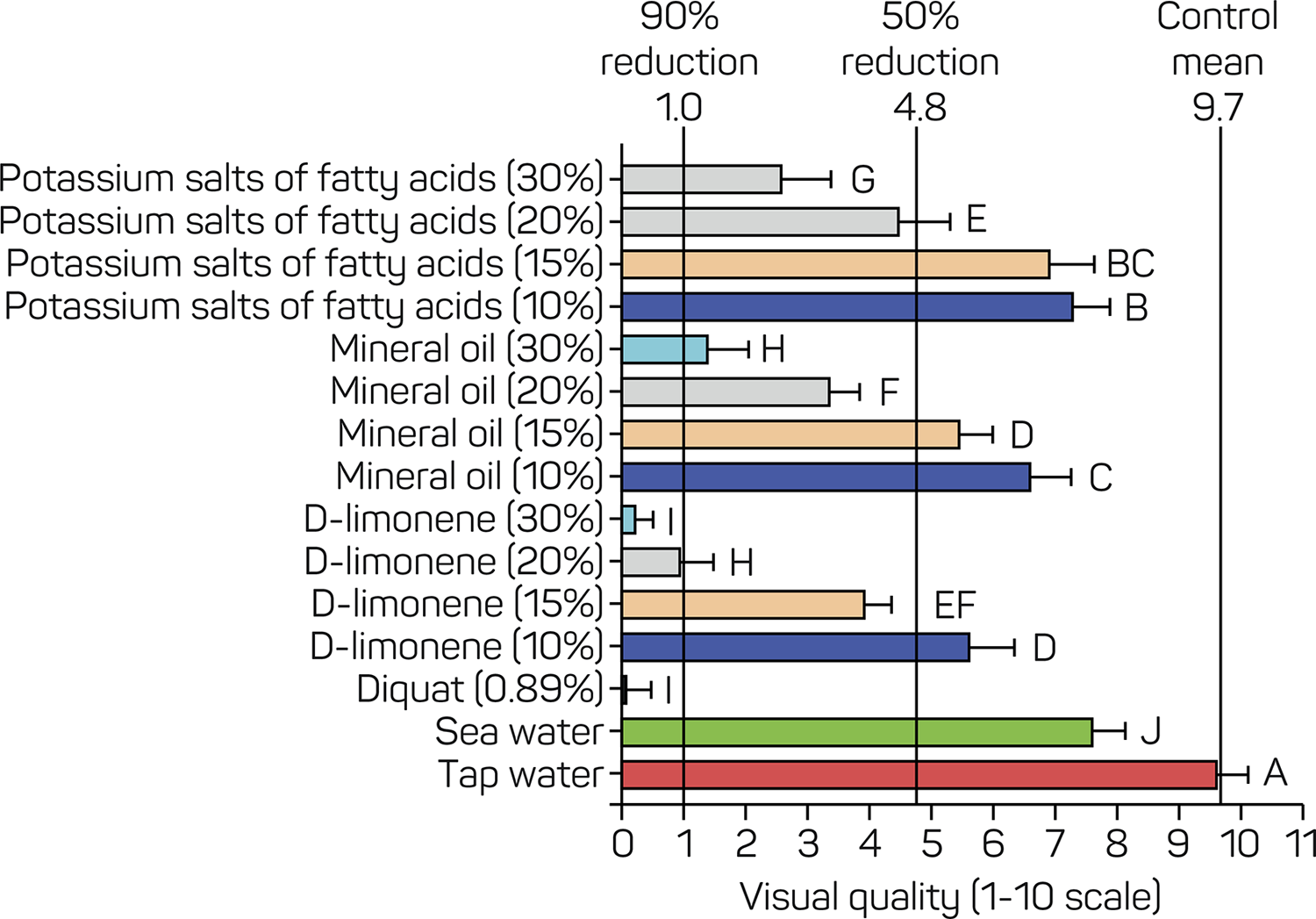
ANOVA results indicated significant variations among treatments on their effect on treated plant visual quality (F13, 266 = 510.390, P < 0.0001) and dry biomass weight (F13, 266 = 331.736, P < 0.0001). Diquat (0.89%) proved most effective, with the lowest visual quality recorded (0.2500). D-limonene at a 30% concentration achieved comparable results to diquat (0.89%) (>90% reduction compared to the control), with an average visual quality score of 0.45. Mineral oil and potassium salts of fatty acids at 20% and above showed significant reductions in dry biomass compared to the control. Additionally, the treatments d-limonene (20%) and mineral oil at 20 and 30% reduced P. stratiotes treated plants visual quality to >50%, five days after treatment compared to the control (Figure 1). The dry biomass weight of P. stratiotes treated plants was reduced by most single-product treatments compared to the untreated control. Diquat (0.89%) and d-limonene at 30% and 20% achieved a 90% reduction, while d-limonene (15%), mineral oil (30% and 20%), and potassium salts of fatty acids (30%) reduced dry biomass weight by more than 50% (Figure 2). These findings suggest varying levels of effectiveness among treatments, with diquat and d-limonene demonstrating the greatest efficacy.
Visual quality of Pistia stratiotes plants 5 weeks after single-product treatment. A numerical scale of 0 through 10 is used to describe visual quality, where 0 represents dead; 5 denotes fair quality, acceptable, somewhat desirable form and color, with little to no chlorosis or necrosis; and 10 indicates excellent quality, perfect condition, healthy and robust, with excellent color and form. Bars represent the mean of 20 replicates. Treatments labeled with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
Biomass of Pistia stratiotes plants 5 weeks after single-product treatment. Bars are the mean of 20 replicates. Treatments coded with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
3.1.2 Impact of applying d-limonene, mineral oil, and potassium salts of fatty acids (prepared with sea water) on the population density of Pistia stratiotes
Statistical analyses revealed significant variations among treatments on their effect on treated plant visual quality (F14, 285 = 422.612, P < 0.0001) and dry biomass weight (F14, 285 = 385.941, P < 0.0001). Based on visual quality results, the only single-product treatments that provided more than 90% reduction compared to the control were diquat (0.89%) and d-limonene at 20 and 30% concentration. D-limonene (15%), mineral oil at 20 and 30%, and potassium salts of fatty acids at 20 and 30% reduced water lettuce visual quality by > 50%, five days after treatment. All the other tested treatments had an effect on waterlettuce visual quality compared to control, but did not reach 50% (Figure 3). Pistia stratiotes plant dry biomass weight was reduced by all single-product treatments compared with untreated control plants, with diquat (0.89%) and d-limonene at 20 and 30%, mineral oil at 30%, and potassium salts of fatty acids at 30% reducing dry biomass weight by >90% (Figure 4). All tested treatments, except seawater and mineral oil plus potassium salts of fatty acids at 10% concentration, reduced dry biomass weight by >50% (Figure 4).
Visual quality of Pistia stratiotes plants 5 weeks after single-product treatment. A numerical scale of 0 through 10 is used to describe visual quality, where 0 represents dead; 5 denotes fair quality, acceptable, somewhat desirable form and color, with little to no chlorosis or necrosis; and 10 indicates excellent quality, perfect condition, healthy and robust, with excellent color and form. Bars represent the mean of 20 replicates. Treatments labeled with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
Biomass of Pistia stratiotes plants 5 weeks after single-product treatment. Bars are the mean of 20 replicates. Treatments coded with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
3.1.3 Impact of simultaneous application of d-limonene, mineral oil, and potassium salts of fatty acids (prepared with tap water) on the population density of Pistia stratiotes
Statistical analyses indicated significant variations among treatments on their effect on treated plant visual quality (F13, 266 = 459.065, P < 0.0001) and dry biomass weight (F13, 266 = 402.084, P < 0.0001). The most promising combinations to control P. stratiotes were diquat (0.89%), d-limonene + mineral oil (20%), d-limonene + mineral oil (30%), d-limonene + potassium salts of fatty acids (30%), and mineral oil + potassium salts of fatty acids (30%); these treatments reduced visual quality (Figure 5), and dry biomass weight by >90% (Figure 6). The d-limonene + potassium salts of fatty acids (30%) treatment also reduced dry biomass weight by >90% compared with untreated control plants. Only d-limonene + potassium salts of fatty acids (10%) and mineral oil + potassium salts of fatty acids (10%) failed to reduce dry biomass weight by >50% compared with untreated control plants. Similarly, the latter treatments with mineral oil + potassium salts of fatty acids (10%) treatment failed to reduce visual quality by >50%.
Visual quality of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids. A numerical scale of 0 through 10 is used to describe visual quality, where 0 represents dead; 5 denotes fair quality, acceptable, somewhat desirable form and color, with little to no chlorosis or necrosis; and 10 indicates excellent quality, perfect condition, healthy and robust, with excellent color and form. Bars represent the mean of 20 replicates. Treatments labeled with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
Biomass of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids. Bars are the mean of 20 replicates. Treatments coded with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
3.1.4 Impact of simultaneous application of d-limonene, mineral oil, and potassium salts of fatty acids (prepared with sea water) on the population density of Pistia stratiotes
Statistical analyses revealed significant effects of the treatments on both visual quality (F14, 285 = 726.191, P < 0.0001) and dry biomass weight (F14, 285 = 779.849, P < 0.0001) of treated P. stratiotes plants. All the tested treatments successfully reduced visual quality (Figure 7), and dry biomass weight by >50% (Figure 8). Only 4 of the 15 treatment combinations failed to reduce dry biomass weight by >90% compared with untreated control plants, and only 6 combinations failed to reduce visual quality by >90% compared to the untreated plants.
Visual quality of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids. A numerical scale of 0 through 10 is used to describe visual quality, where 0 represents dead; 5 denotes fair quality, acceptable, somewhat desirable form and color, with little to no chlorosis or necrosis; and 10 indicates excellent quality, perfect condition, healthy and robust, with excellent color and form. Bars represent the mean of 20 replicates. Treatments labeled with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
Biomass of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids. Bars are the mean of 20 replicates. Treatments coded with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
3.2 Greenhouse trials
ANOVA results indicated significant variations among treatments in their effects on treated plant visual quality (F5, 114 = 717.525, P < 0.0001) and dry biomass weight (F5, 114 = 473.383, P < 0.0001). All the tested treatments except the seawater treatment reduced visual quality (Figure 9), and dry biomass weight by >90% (Figure 10) compared to the tap water control. Visual quality was reduced by >50% in plants treated with seawater only.
Visual quality of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids under greenhouse conditions. A numerical scale of 0 through 10 is used to describe visual quality, where 0 represents dead; 5 denotes fair quality, acceptable, somewhat desirable form and color, with little to no chlorosis or necrosis; and 10 indicates excellent quality, perfect condition, healthy and robust, with excellent color and form. Bars represent the mean of 20 replicates. Treatments labeled with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
Biomass of Pistia stratiotes plants 5 weeks after treatment with combinations of D-limonene, mineral oil, and potassium salts of fatty acids under greenhouse conditions. Bars are the mean of 20 replicates. Treatments coded with the same letter are not different at P = 0.05. The right bold vertical line indicates the mean of control plants (tap water), whereas the central and left bold vertical lines indicate 50% and 90% reductions compared with control plants
The results of the laboratory and greenhouse trials provide comprehensive insights into the efficacy of various treatments for managing P. stratiotes. This invasive aquatic plant poses significant ecological and economic threats, making effective control measures essential.
Diquat (0.89%) emerged as to was the most effective single-product treatment in both laboratory and greenhouse trials, consistently achieving over 90% reduction in both visual quality and dry biomass weight of P. stratiotes. This finding aligns with previous studies that highlight the potency of diquat in controlling aquatic weeds (Martins et al., 2002; Mudge, Haller, 2012). Notably, d-limonene, a natural product, demonstrated promising efficacy, particularly at concentrations of 20% and 30%, where it achieved comparable results to diquat. These concentrations and their effectiveness on waterlettuce are consistent with the findings of Gettys et al. (2021). This finding underscores the potential of natural alternatives for weed control, offering environmentally friendly options for management practices while potentially reducing reliance on synthetic herbicides (Invasive Species Specialist Group, 2005; Rojas-Sandoval et al., 2013). Previous studies have highlighted d-limonene’s potential for selective weed management without harming desirable native plants (Emerine, 2010; Mudge, Haller, 2012; Glomski, Mudge, 2013). However, its high application cost compared to traditional herbicides may limit broad-scale deployment (Gettys et al., 2021). The reduction in visual quality and dry weight is likely due to the impact of the treatments on the plant’s growth and reproductive processes, as observed in similar studies (Ajuonu et al., 2003; Gettys et al., 2021). The simultaneous application of different products yielded promising results, especially in greenhouse trials. Combinations such as d-limonene + mineral oil and d-limonene + potassium salts of fatty acids demonstrated substantial reductions in both visual quality and dry biomass weight of P. stratiotes. These findings highlight the synergistic effects that can be achieved by combining multiple control methods (Cilliers et al., 1996; Gettys et al., 2021). The use of potassium salts of fatty acids can help reduce plant growth by increasing the salinity of the aquatic environment; however, this increase in salinity may not be desirable in all contexts, as it can adversely affect non-target organisms and the overall ecosystem, making it essential to consider the specific environmental conditions and potential impacts before applying these treatments, while mineral oil can create a film on the water’s surface, limiting photosynthesis and thus suffocating the plant, and it is not recommended if there is a possibility of the water being used for drinking purposes (Service, 1962). By leveraging the strengths of different products, enhanced efficacy can be achieved while potentially reducing the risk of resistance development and environmental impact.
Variations in temperature and humidity throughout the study period reflect typical weather patterns, which may affect the growth and reproduction of P. stratiotes as well as the efficacy of the treatments. These environmental factors could affect the performance of the natural products tested and should be taken into account in future research and practical applications (Queensland Government, 2017; Millane, Caffrey, 2014). Despite the promising results, several challenges and considerations need to be addressed. The persistence of P. stratiotes, facilitated by vegetative reproduction and seed longevity, underscores the need for sustained and integrated management efforts (Millane, Caffrey, 2014). Additionally, the cost of adopting alternative control methods, such as natural products, must be carefully evaluated. While these products may offer environmentally friendly alternatives, their higher costs compared to conventional herbicides could pose challenges for widespread adoption, particularly in large-scale management programs (Center, 1994). Gettys et al. (2021) reported that using a combination of 20% d-limonene + 10% acetic acid (the most cost-effective treatment) instead of diquat for waterlettuce management would result in a 22-fold increase in material costs. Thus, a cost-benefit analysis is crucial to determine the feasibility and sustainability of implementing these control strategies on a broader scale.
4.Conclusions
In conclusion, the findings of this study highlight the effectiveness of diverse strategies in controlling P. stratiotes infestations. Both single-product treatments and combinations of products have shown promise in reducing the population density and biomass of P. stratiotes. Single-product treatments, such as specific herbicides or natural compounds like d-limonene, offer targeted control measures, while combinations of products, such as d-limonene with mineral oil or potassium salts of fatty acids, provide synergistic effects that enhance overall efficacy. However, while these findings are encouraging, further research is warranted to comprehensively assess the efficacy, cost-effectiveness, and environmental impact of these control methods. Integrated approaches that combine chemical, physical, and biological control methods have emerged as particularly effective in addressing P. stratiotes infestations. By harnessing the strengths of each approach, these integrated strategies offer a multifaceted and sustainable solution for managing P. stratiotes, thereby mitigating its ecological and economic impacts.
Acknowledgements
No applicable.
References
- Ajuonu O, Schade V, Veltman B, Sedjro K, Neuenschwander P. Impact of the weevils Neochetina eichhorniae and N. bruchi (Coleoptera: Curculionidae) on water hyacinth, Eichhornia crassipes (Pontederiaceae), in Benin, West Africa. Afr Entomol. 2003;11(2):153-61.
- Anderson L. Potential for sediment-applied acetic acid for control of invasive Spartina alterniflora J Aquat Plant Manag. 2007;45(2):100-5.
-
Baker JM. The effects of oils on plants. Environ Pollut. 1970;1(1):27-44. Available from: https://doi.org/10.1016/0013-9327 (70)90004-2
» https://doi.org/10.1016/0013-9327 (70)90004-2 -
Bradfield Industries. Horticultural vinegar supplemental info: 20% Acetic acid. Springfield: Bradfield Industries; 2019[access May 15, 2019]. Available from: https://www.bradfieldind.com/vinegar.htm
» https://www.bradfieldind.com/vinegar.htm -
Center TD. Biological control of weeds: waterhyacinth and waterlettuce. In: Rosen D, Bennett FD, Capinera JL, editors. Pest management in the tropics: biological control: a Florida perspective. Andover: Intercept Limited; 1994[access Oct 5, 2021]. p. 481-521. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19951106929
» https://www.cabidigitallibrary.org/doi/full/10.5555/19951106929 - Cilliers CJ, Zeller D, Strydom D. Short- and long-term control of water lettuce ( P. stratiotes ) on seasonal water bodies and on a river system in the Kruger National Park, South Africa. In: Caffrey JM, Barrett PRF, Murphy KJ, Wade PM, editors. Management and ecology of freshwater plants: proceedings of the 9th International Symposium on Aquatic Weeds. Berlin: Springer Science+Business Media; 1996. p. 173-9.
-
Clark R, Dew A. Annual report of pollutant discharges to the surface waters of the state from the application of pesticides. Tallahassee: Florida Fish and Wildlife Conservation Commission; 2019[access Oct 5, 2021]. Available from: https://myfwc.com/media/19111/npdes-2018.pdf
» https://myfwc.com/media/19111/npdes-2018.pdf -
Cordo HA, Sosa A. The weevils Argentinorhynchus breyeri, A. bruchi and A. squamosus (Coleoptera: Curculionidae), candidates for biological control of waterlettuce (Pistia stratiotes). In: Spencer NR, editor. Proceedings of the X international symposium. Bozeman: Montana State University; 1999[access Dec 15, 2015]. p. 325-35. Available from: http://www.invasive.org/publications/xsymposium/proceed/05pg325.pdf
» http://www.invasive.org/publications/xsymposium/proceed/05pg325.pdf -
Cutelle MA, Armel GR, Brosnan JT, Kopsell DA, Klingeman WE, Flanagan PC et al. Evaluation of container ornamental species tolerance to three p-hydroxyphenylpyruvate dioxygenase-inhibiting herbicides. Hort Technol. 2013;23(3):319-24. Available from: https://doi.org/10.21273/HORTTECH.23.3.319
» https://doi.org/10.21273/HORTTECH.23.3.319 -
Domenghini JC. Comparison of acetic acid to glyphosate for weed sup pression in the garden. Hort Technol. 2020;30(1):82-7. Available from: https://doi.org/10.21273/HORTTECH04453-19
» https://doi.org/10.21273/HORTTECH04453-19 - Emerine SE. Greenhouse response of six aquatic invasive weeds to imazamox. J Aquat Plant Manag. 2010;48:105-11.
-
European and Mediterranean Plant Protection Organization. Organisation Europénne et Méditerranéenne pour la Protection des Plantes. Pistia stratiotes L. Bull OEPP/EPPO. 2017;47(3):537-43. Available from: https://doi.org/10.1111/epp.12429
» https://doi.org/10.1111/epp.12429 -
Florida Fish and Wildlife Conservation Commission - FWC. Annual report of pollutant discharges to the surface waters of the state from the application of pesticides 1 Jan. 2018 through 31 Dec. 2018. Tallahassee: Florida Fish and Wildlife Conservation Commission; 2019[access Mar 20, 2019]. Available from: https://myfwc.com/media/19111/npdes-2018.pdf
» https://myfwc.com/media/19111/npdes-2018.pdf -
Gettys LA, Haller WT. Effect of herbicide-treated irrigation water on four vegetables. Weed Technol. 2012;26(2):272-8. Available from: https://doi.org/10.1614/WT-D-11-00120.1
» https://doi.org/10.1614/WT-D-11-00120.1 -
Gettys LA, Moore KA. Greenhouse production of native aquatic plants. Hort Technol. 2019;29(1):41-5. Available from: https://doi.org/10.21273/HORTTECH04212-18
» https://doi.org/10.21273/HORTTECH04212-18 -
Gettys LA, Thayer KL, Sigmon JW, Bishop JH. Selectivity and efficacy of acetic acid and d-limonene on four aquatic plants. Hort Technol. 2023;33(2):186-92. Available from: https://doi.org/10.21273/HORTTECH05168-22
» https://doi.org/10.21273/HORTTECH05168-22 -
Gettys LA, Thayer KL, Sigmon JW. Evaluating the effects of acetic acid and d-limonene on four aquatic plants. Hort Technol. 2021;31(2):225-33. Available from: https://doi.org/10.21273/HORTTECH04769-20
» https://doi.org/10.21273/HORTTECH04769-20 -
Gettys LA, Thayer KL, Sigmon JW. Phytotoxic effects of acetic acid and d-limonene on four aquatic plants. Hort Technol. 2022;32(2):110-8. Available from: https://doi.org/10.21273/HORTTECH04986-21
» https://doi.org/10.21273/HORTTECH04986-21 - Glomski LM, Mudge CR. Effect of subsurface and foliar applications of bispyribac-sodium on water hyacinth, water lettuce, and giant Salvinia. J Aquat Plant Manag. 2013;51:62-5.
- Haller WT, Gettys LA. Pond, selectivity and irrigation studies on potential new aquatic herbicides. Aquatics. 2013;35(2):6-10.
-
Hill MP. The impact and control of alien aquatic vegetation in South African aquatic ecosystems. Afr J Aquat Sci. 2003;28(1):19-24. Available from: https://doi.org/10.2989/16085914.2003.9626595
» https://doi.org/10.2989/16085914.2003.9626595 -
Hussner A. Long-term macrophyte mapping documents a continuously shift from native to non-native aquatic plant dominance in the thermally abnormal River Erft (North Rhine-Westphalia, Germany). Limnologica. 2014;48:39-45. Available from: https://doi.org/10.1016/j.limno.2014.05.003
» https://doi.org/10.1016/j.limno.2014.05.003 -
Invasive Species Specialist Group - ISSG. Pistia stratiotes. Global Invasive Species Database. 2005[access Sept 2015]. Available from: https://www.iucngisd.org/gisd/speciesname/Pistia+stratiotes
» https://www.iucngisd.org/gisd/speciesname/Pistia+stratiotes -
Koschnick TJ, Haller WT, MacDonald GE. Turf and ornamental plant tolerances to endothall in irrigation water: I ornamental species. Hort Technol. 2005;15:318-23. Available from: https://doi.org/10.21273/HORTTECH.15.2.0318
» https://doi.org/10.21273/HORTTECH.15.2.0318 -
Martins D, Velini ED, Negrisoli E, Tofoli GR. Chemical control of Pistia stratiotes, Echhornia crassipes and Salvinia molesta in reservoirs. Planta Daninha. 2002;20(spe):83-8. Available from: https://doi.org/10.1590/S0100-83582002000400010
» https://doi.org/10.1590/S0100-83582002000400010 - Mennema J. [Is waterlettuce ( Pistia stratiotes L.) becoming a new aquatic weed in the Netherlands?] Natura. 1977;74:187-90. Dutch
-
Millane M, Caffrey J. Risk assessment of P. stratiotes . Dublin: Inland Fisheries Ireland; 2014[access Oct 25, 2016]. Available from: https://nonnativespecies.ie/wp-content/uploads/2014/03/Pistia-stratiotes-Water-Lettuce1.pdf
» https://nonnativespecies.ie/wp-content/uploads/2014/03/Pistia-stratiotes-Water-Lettuce1.pdf - Mudge CR, Haller WT. Response of target and nontarget floating and emergent aquatic plants to flumioxazin. J Aquat Plant Manag. 2012;50:111-6.
- Mudge CR, Koschnick TJ, Haller WT. Ornamental plant susceptibility to diquat in overhead irrigation water. J Aquat Plant Manag. 2007;45:40-3.
- Quarles W. Alternative herbicides in turfgrass and organic agriculture. IPM Pract. 2010;32(5/6):1-8.
-
Queensland Government. Water lettuce. Brisbane: Queensland Government; 2017[access Oct 25, 2016]. Available from: https://www.business.qld.gov.au/
» https://www.business.qld.gov.au/ -
Rojas-Sandoval J, Acevedo-Rodríguez P, Mikulyuk A. Pistia stratiotes (water lettuce). CABI Compendium. Aug 30, 2013[access July 23, 2016]. Available from: http://www.cabi.org/isc/datasheet/41496
» http://www.cabi.org/isc/datasheet/41496 -
Saha NC, Bhunia F, Kaviraj A. Comparative toxicity of three or ganic acids to freshwater organisms and their impact on aquatic ecosystems. Human Ecol Risk Assess. 2006;12(1):192-202. Available from: https://doi.org/10.1080/10807030500430625
» https://doi.org/10.1080/10807030500430625 -
Service MW. The possible control of Pistia stratiotes L. with diquat. Trans R Soc Trop Med Hyg. 1962;56(6):529-32. Available from: https://doi.org/10.1016/0035-9203 (62)90078-0
» https://doi.org/10.1016/0035-9203 (62)90078-0 -
Shrestha AM, Moretti M, Mourad N. Evaluation of thermal implements andorganic herbicides for weed control in a nonbearing almond ( Prunus dulcis ) or chard. Weed Technol. 2012;26(1):110-6. Available from: https://doi.org/10.1614/WT-D-11-00083.1
» https://doi.org/10.1614/WT-D-11-00083.1 -
Smith HC, Ferrell JA, Koschnick TJ. Flurprimidol performance on ornamental species in relation to trimming time and method of applica tion. HortScience. 2014;49:1305-8. Available from: https://doi.org/10.21273/HORTSCI.49.10.1305
» https://doi.org/10.21273/HORTSCI.49.10.1305 -
Smith-Fiola D, Gill S. Vinegar: an alternative to glyphosate? College Park: University of Maryland Extension; 2017[access Dec 12, 2018]. Available from: https://extension.umd.edu//sites/extension.umd.edu/files/_docs/programs/ipmnet/Vinegar-AnAlternativeToGlyphosate-UMD-Smith-Fiola-and-Gill.pdf
» https://extension.umd.edu//sites/extension.umd.edu/files/_docs/programs/ipmnet/Vinegar-AnAlternativeToGlyphosate-UMD-Smith-Fiola-and-Gill.pdf - Stubbs D, Layne CR. Requirements for registration of aquatic herbicides. In: Gettys LA, Haller WT, Petty DG editions. Biology and control of aquatic plants: a best management practices handbook. 4th ed. Marietta: Aquatic Ecosystem Restoration Foundation; 2020. p. 155-162.
-
Tootoonchi M, Gettys LA, Thayer KL, Markovich IJ, Sigmon JW, Sadeghibaniani S. Ecotypes of aquatic plant Vallisneria americana tolerate different salinity concentrations. Diversity. 2020;12(2):1-16. Available from: https://doi.org/103390/d12020065
» https://doi.org/103390/d12020065 -
Webber III CL, White Jr PM, Shrefler JW, Spaunhorst D. Impact of acetic acid concentration, application volume, and adjuvants on weed control efficacy. J Agr Sci. 2018;10(8):1-6. Available from: https://doi.org/10.5539/jas.v10n8p1
» https://doi.org/10.5539/jas.v10n8p1
-
Funding:
This work was carried out within the framework of a partnership agreement between the Bouregreg and Chaouia Hydraulic Basin Agency (ABHBC) and The National Institute of Agricultural Research (INRA). The purpose of the agreement is a research project on developing methods to control Pistia stratiotes L. in the Koreima Dam reservoir and other bodies of water within the ABHBC’s area of operation.
Edited by
-
Approved by:
Editor in Chief: Carol Ann Mallory-SmithAssociate Editor: Chandrima Shyam
Publication Dates
-
Publication in this collection
17 Mar 2025 -
Date of issue
2025
History
-
Received
2 May 2024 -
Accepted
4 Jan 2025

 Evaluating natural product-based herbicides for effective control of invasive water lettuce (Pistia stratiotes L.)
Evaluating natural product-based herbicides for effective control of invasive water lettuce (Pistia stratiotes L.)