ABSTRACT.
In tropical and subtropical regions, nitrogen (N) is often limited and significantly impacts corn production costs. In this context, bio-inputs have been used to reduce N and water supplied to plants. This study assesses the impact of varying N levels, Azospirillum brasilense seed inoculation, and biostimulant use on the agronomic performance of summer-grown green corn (Zea mays L.) across three growing seasons (2017/2018, 2018/2019, and 2019/2020). Five N-fertilizer levels (0, 30, 60, 90, and 120 kg N ha-1), two A. brasilense seed inoculation levels (0 and 100 mL ha-1), and two biostimulant doses (0.0 and 1.0 L ha-1) were evaluated in a completely randomized block design, arranged in a 5 x 2 x 2 factorial scheme, with four replications. Results showed that A. brasilense seed treatment did not increase ear yield or kernel protein content (PROT) and reduced PROT when combined with the two highest N levels. The application of biostimulant increased ear weight by 5.08% in the 2018/2019 growing season, leading to an increase in PROT. However, the use of inoculant and biostimulant did not reduce the amount of N-fertilizer applied to green corn plants.
Keywords:
anti-stress effect; nitrogen levels; plant biostimulants; plant growth-promoting bacteria; Zea mays L
Introduction
Brazil, the world's third-largest corn producer following the United States and China (FAO, 2023), benefits from favorable soil and climate conditions for corn production, including green ears used for human consumption and by-products. Corn and other non-leguminous crops have a substantial nitrogen (N) demand (Galindo et al., 2019) since N is integral to various plant components, including chlorophyll, proteins, hormones, enzymes, nucleic acids, and secondary metabolites (De-Bang, Husted, Laursen, Persson, & Schjoerring, 2021).
Nonetheless, tropical soils often suffer from low N availability, limiting crop yields (Martins et al., 2018). Consequently, N-fertilizers are commonly employed to meet these nutritional requirements (Galindo et al., 2019). Unfortunately, improper fertilizer use leads to environmental issues like water eutrophication and air pollution from ammonia (NH3), nitrates (NO3 -), and nitrous oxide (N2O) emissions, resulting in low N use efficiency (Tei, De Neve, de Haan, & Kristensen, 2020).
In recent years, a strategy to mitigate negative fertilizer effects involves using associative diazotrophic bacteria, particularly Azospirillum brasilense. This species positively influences plant growth through biological N fixation (BNF), increased hormone synthesis, and pest control mechanisms (Fukami, Cerezini, & Hungria, 2018). A. brasilense has also been found to increase proline content in roots and shoots, improve water potential, increase apoplectic pathways for water content, augment cell wall elasticity, photosynthetic pigments, and photoreceptors, and decrease stomatal conductance and transpiration, especially under drought stress (Ngumbi & Kloepper, 2016).
Another sustainable approach for managing N fertilizer and increasing yields is the use of plant biostimulants (Tei et al., 2020). These products offer a reduced carbon footprint and contribute to mitigating climate change, a crucial concern related to agricultural activities (Singh et al., 2018). Furthermore, biostimulants in corn crops improve drought resistance, photosynthetic activity, biomass production, and grain yield (Shemi et al., 2021).
Despite numerous studies on diazotrophic bacteria and biostimulants, limited research evaluates their combined impact on N fertilization in corn crops. This study hypothesizes that Azospirillum brasilense inoculation, in combination with a biostimulant, can enhance drought tolerance and reduce the need for mineral N for optimal green corn production. Thus, this study aimed to assess the effects of different N levels in conjunction with A. brasilense and a biostimulant on the agronomic traits of green corn during summer growing seasons.
Material and methods
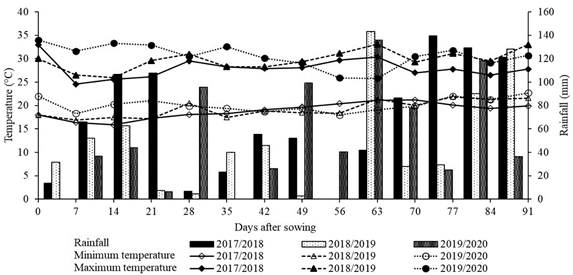
Field experiments were conducted at the Iguatemi Experimental Farm (FEI), which is part of the State University of Maringá (UEM). The farm is situated in the Iguatemi District, Maringá, Paraná State, Brazil (23º20'48" S and 52º04'17" W; altitude of 550 m). The climate in this region is classified as Cfa (subtropical) by Köppen, characterized by dry winters, hot summers, and abundant rainfall (IAPAR, 1994). Figure 1 shows the recorded rainfall data and temperatures during the experiments.
Records of rainfall, and minimum and maximum temperatures, obtained from the FEI Seed Analysis Laboratory, for the 2017/2018, 2018/2019, and 2019/2020 summer growing seasons, Maringá, Paraná State, Brazil.
The soil in the experimental area was identified as dystroferric red Nitosol (Santos et al., 2018), with clayey texture (520 g kg-1 clay, 140 g kg-1 silt, and 340 g kg-1 sand). Soil samples collected at a depth of 0.0 to 0.20 m in the three growing seasons had the following average properties: pH = 5.06; C = 11.99 g kg-1; P = 15.23 mg dm-3; K+ = 0.45 cmolc dm-3; Ca+2 = 3.84 cmolc dm-3; Mg+2 = 1.28 cmolc dm-3; Al+3 = 0.00 cmolc dm-3.
The study used a randomized complete block design in a 5 x 2 x 2 factorial scheme with four replications, resulting in 20 treatments and 80 experimental units. The treatments included five nitrogen fertilizer levels (0, 30, 60, 90, and 120 kg N ha-1), with 30 kg N ha-1 applied at sowing and the remainder as topdressing at the V4 growth stage (Ritchie, Hanway, & Benson, 1993). Two levels of seed inoculation (0 and 100 mL ha-1) with Azospirillum brasilense-based liquid inoculant (Ab-V5 and Ab-V6 strains) at 2 x 108 colony forming units (CFU) mL-1 and two foliar applications of biostimulants (0.0 and 1.0 L ha-1) were also considered. The biostimulant composition included cytokinins, betaine glycines, amino acids, in addition to 3% N, 17% phosphorous pentoxide (P2O5), 2.5% manganese (Mn), 5.7% zinc (Zn), 1.8% total organic carbon (TOC), and 19.3% algae extracts.
The experimental plots were 6.0 m long and consisted of five rows of plants spaced 0.9 m apart, totaling 27 m2. Data were collected from the central three rows, excluding 0.5 m at each end, resulting in a useful area of 13.5 m2. The AG 1051 hybrid was used in all three growing seasons, planted on June 6th, following a no-till system on black oak mulch (Avena strigosa Schreb.). Plants were desiccated with 2.5 L ha-1 glyphosate herbicide (480 g a.i. L-1) 10 days before planting when cover plants were at flowering.
Green corn seeds were industrially treated with (0.8 mL kg-1 of seeds) and pirimiphos -methyl-based (0.08 mL kg-1 of seeds) insecticides and fludioxonil + metalaxyl-M-based (1.5 mL kg-1 of seeds) fungicides. And, immediately before sowing, these seeds were manually inoculated with A. brasilense in plastic bags, using the recommended dose (100 mL ha-1). The seeds were thoroughly turned over until they were fully coated with the inoculant.
After being opened, furrows were applied with fertilizer containing phosphorous (P) and potassium (K) distributed using a tractor seeder. Before sowing, all treatments received 250 kg ha-1 of the 0-8-16 (NPK) formula and, at sowing, urea [CO(NH2)2] was applied as nitrogen fertilizer (46% N) after manual application of 30 kg N ha-1 only in treatments receiving this nutrient.
The AG 1051 hybrid was sown using a hand-held seed planter on 10/20/2017, 10/19/2018, and 10/18/2019 at a seeding rate of 15 seeds m-1 and row spacing of 0.9 m. When plants reached the V2 growth stage (Ritchie et al., 1993), thinning was performed to remove less vigorous individuals, resulting in a final population of 55,555 plants ha-1, or 5 plants m-1.
Urea [CO (NH2)2] was manually applied as a nitrogen fertilizer when at least 50% of the plants reached the V4 stage (Ritchie et al., 1993), with varying application rates corresponding to the specific treatment (60, 90, and 120 kg N ha-1). The N fertilizer was applied to the soil surface at approximately 0.10 m away from plant rows to avoid direct contact between fertilizer and corn leaves. Afterwards, the area was irrigated with about 10 mm water depth to improve fertilizer incorporation into the soil.
The biostimulant was administered to corn leaves at the V4 growth stage (Ritchie et al., 1993), following the manufacturer's recommended dose of 1.0 L ha-1. This application was carried out using a CO2 pressurized backpack sprayer equipped with five spray nozzles, operating at a constant pressure of 200,000 Pa and a flow rate of 3.5 × 10-5 m-3 s-1. The sprayer was positioned at a height of 0.5 m from the target, with a speed of 1 m s-1, covering an area 2.5 m2 and applying a solution volume of 140 L ha-1. To prevent drift and reduce contamination in treatments without biostimulant, application was conducted under wind speeds below 2.78 m s-1.
Once the crop was established and immediately after weed emergence, herbicides were applied, including tembotrione at a rate of 100 g a.i. ha-1 and atrazine at a rate of 1,500 g a.i. ha-1. These herbicides were combined with a mineral oil-based adjuvant at a rate of 756 g a.i. ha-1 and mixed in a tank for application. Additionally, manual weeding was performed as required to ensure that weeds did not adversely affect the agronomic performance of corn plants.
To control insect pests, imidacloprid + beta-cyfluthrin (100 g + 12.5 g a.i. ha-1) was applied to combat the green-belly stink bug, Dichelops melacanthus (Dallas) (Hemiptera: Pentatomidae), and lufenuron (15 g a.i. ha-1) and methomyl (129 g a.i. ha-1) for the farm armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and corn earworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae). During the three growing seasons, there were no observed instances of disease that necessitated phytosanitary control measures.
To assess plant growth at the peak bloom stage (VT) (Ritchie et al., 1993), the following measurements were taken: i) plant height was determined by measuring the length (m) from the soil surface to the base of the tassel. Measurements were collected from ten randomly selected plants within the study area of each experimental plot; ii) leaf area index (LAI) was calculated using the methodology proposed by Francis, Rutger, and Palmer (1969). This involved measuring the length (L) and maximum width (W) of all leaves with a green area greater than 50%. Five plants were randomly selected from the study area of each plot. The leaf area (LA) was calculated using the formula: LA = 0.75 x L x W. The LAI was subsequently determined using the expression: LAI = LA / (e1 x e2), where e1 and e2 represent the spacing between plants in the row (m) and between rows (m), respectively.
Upon the onset of styles and stigmas on the ears, denoted as stage R1 for green corn (Ritchie et al., 1993), we collected ten leaf samples from the study area of each plot. These samples were taken both below and opposite the main spike to analyze the total leaf N content. Chemical analysis was performed following the method outlined by Cantarella, van Raij, and Camargo (1997), using the Kjeldahl method.
Ears were manually harvested from each study area when the plants reached stage R3 (Ritchie et al., 1993), during the second half of January in the years 2018, 2019, and 2020. In each growing season, after dehusking and weighing the collected ears, the yield was determined and extrapolated to provide yield values in metric tons per hectare (Mg ha-1).
Furthermore, a selection process was carried out to isolate commercial ears that met specific criteria: their length and diameter exceeded 0.15 and 0.03 m, respectively, and they were free from damage caused by insect pests and diseases, as recommended by Albuquerque, Von Pinho, Borges, Souza Filho, and Fiorini (2008). These commercial ears were weighed to determine the dehusked commercial ear yield in Mg ha-1.
To quantify the raw protein content, five ears were randomly selected from each study area. The kernels were extracted, dried in a forced air circulation oven at a temperature of 56ºC, and analyzed using the Kjeldahl method (Brasil, 2005).
The experimental data from each growing season underwent separate assessments through the Shapiro-Wilk test (p > 0.01) to evaluate the normality of errors and the Levene test (p > 0.01) to check for homoscedasticity of variances. Subsequently, individual analysis of variance (p < 0.05) was conducted to determine if the ratios between mean square residuals met the criterion of being lower than 7:1, following the guidelines of Banzatto and Kronka (2006). Under these conditions, the collected data were subjected to a joint analysis of variance, and significant findings were further subjected to statistical breakdown.
The impact of nitrogen fertilizer levels was explored through polynomial regression analysis. The effects of seed inoculation with A. brasilense and application of biostimulants were assessed using the F-test within the analysis of variance, comparing two factor levels. For the effects attributed to different growing seasons, treatment means were subjected to Tukey's multiple comparison test (p < 0.05). All statistical analyses were performed using the SISVAR program (Ferreira, 2014).
Results and discussion
The climate data depicted in Figure 1 reveals that the total rainfall for the green corn growing seasons of 2017/2018, 2018/2019, and 2019/2020 amounted to 897.0, 523.6, and 689.1 mm, respectively. Nevertheless, despite the overall adequacy of rainfall levels for the crop, the presence of poor rainfall distribution and extended periods of drought during the dry season has led to drought stress, thereby impacting corn growth and yield (Nemeskéri, Molnár, Rácz, Dobos, & Helyes, 2019).
It is noteworthy that rainfall distribution was notably more consistent during the 2017/2018 season compared to the 2018/2019 and 2019/2020 seasons (Figure 1). In the latter two years, dry spells occurred precisely when most of the plants were at the VT (tasseling) to R1 (silking) stages, which represents one of the most critical periods for the manifestation of drought stress.
The results of the joint analysis of variance for the assessed agronomic traits are summarized in Table 1. The findings show that nitrogen levels and the specific growing seasons had a significant impact on all the variables analyzed. However, in the case of A. brasilense inoculation and the application of a biostimulant, no significant effects were observed for any of the studied traits.
Joint analysis of variance for degrees of freedom (DF), plant height (PH), leaf area index (LAI), total leaf nitrogen content (LNC), dehusked ear yield (DEY), commercial ear yield (CEY), and kernel protein content (PROT) as a function of nitrogen fertilizer levels, Azospirillum brasilense seed inoculation, and biostimulant in three distinct growing seasons.
The average minimum temperatures recorded during the field experiments (Figure 1) were 19.3, 19.5, and 20.1ºC for the 2017/2018, 2018/2019, and 2019/2020 growing seasons, respectively. Correspondingly, the average maximum temperatures were 27.9, 29.9, and 30.2ºC. Fortunately, no visible damage to the corn crop was observed as a result of temperature fluctuations.
The leaf area index (LAI) exhibited significant differences concerning N levels and growing seasons, as well as inoculation and biostimulant factors. Total leaf nitrogen content (LNC) displayed significant interactions between N levels x growing seasons and N levels x inoculation x biostimulant. Dehusked ear yield (DEY) showed notable interactions between N levels x growing seasons and biostimulant x growing seasons. Meanwhile, kernel protein content (PROT) was significantly influenced by the interactions of N levels x biostimulant and N levels x inoculation x growing seasons (Table 1).
Plant height (PH) and LAI responded positively to varying N fertilizer levels, demonstrating linear increases. In terms of PH, the estimated increase, represented by the angular coefficient, amounted to 0.001 m per kg N ha-1 applied, regardless of other factors tested (Figure 2A). Likewise, irrespective of A. brasilense inoculation and the use of biostimulants, the estimated increases in LAI were 0.008 in the 2017/2018 season and 0.003 per kg N ha-1 applied in the 2018/2019 and 2019/2020 growing seasons (Figure 2B).
Plant height (PH) as a function of nitrogen fertilizer level, Azospirillum brasilense seed inoculation, biostimulant application, and growing seasons (A); leaf area index (LAI) as a function of nitrogen fertilizer level associated with growing seasons, Azospirillum brasilense seed inoculation, and biostimulant application (B). Summer, Maringá, Paraná State, Brazil.
Plants receiving ample nitrogen (N) nourishment demonstrate an enhanced capacity for solar energy absorption, carbon dioxide (CO2) capture, and photosynthetic carbohydrate production (Liu et al., 2019). Consequently, these well-nourished plants tend to accumulate more dry matter due to increased cell division and expansion at growth points (Xu, Fan, & Miller, 2012), resulting in elevated leaf area index (LAI) and plant height (PH). This, in turn, leads to higher ear yield in corn crops (Okumura et al., 2014), as it is intricately linked to the active photosynthetic area of the plants.
Maximum plant height was achieved during the 2017/2018 growing season, exhibiting differences when compared to the other growing seasons (Table 2). In contrast, the leaf area index in the 2017/2018 and 2018/2019 seasons did not differ significantly (p ≥ 0.05) across most of the N levels investigated, and it was notably higher compared to the 2019/2020 season (Table 2).
Leaf area index (LAI) at average Azospirillum brasilense seed inoculation levels and biostimulant application in the growing seasons at each nitrogen fertilizer level; and plant height (PH), under the effect of three growing seasons, at average nitrogen fertilizer levels, seed inoculation and biostimulant application, during summer, in Maringá, Paraná State, Brazil.
In light of these findings, it appears that the higher soil moisture resulting from the rainfall and moderate temperatures during the 2017/2018 growing season may have benefited the green corn crop (Figure 1). The lower daytime and nighttime temperatures observed during the 2017/2018 season likely contributed to reduced respiration (consumption of photoassimilates), favoring net photosynthesis, and resulting in increased plant growth and higher yields (Posch et al., 2019).
Interestingly, irrespective of the N levels and growing seasons, the absence of biostimulant did not result in a statistically significant difference in average LAI with or without inoculation. The observed averages were 3.56 and 3.49, respectively. However, with the addition of biostimulant at the V4 development stage, the use of A. brasilense led to a reduction in LAI by 0.18, from 3.63 to 3.45, which corresponds to a decrease of 5.20%.
According to Fukami et al. (2018), Azospirillum spp. can enhance not only N absorption but also hormone synthesis, which promotes plant growth and delays leaf senescence. This may influence the number of senesced leaves between the end of VT and the onset of R1, consequently affecting LAI. However, the inhibition of A. brasilense effect could be explained by cellular phytotoxicity caused by the considerable number of substances applied to plants, such as the inoculant itself, leaf biostimulant components, insecticides, herbicides, fungicides, and adjuvants. This high concentration of other products might have led to hormonal imbalances, limiting cell division and elongation, which could have resulted in a reduced LAI.
Total leaf nitrogen content (LNC) exhibited significant responses to the second-order interaction among N levels, seed inoculation, and biostimulant (N x I x B) (Figure 3A). This response can be attributed to the influence of biostimulant and Azospirillum brasilense inoculation on N absorption and use. Biostimulants can reduce damage caused by drought stress, increase photosynthetic activity, and boost biomass production (Shemi et al., 2021). On the other hand, seed inoculation with A. brasilense stimulates root system growth in corn plants, thus enhancing nutrient absorption, especially nitrogen (Fukami et al., 2018; Pelloso et al., 2023).
Figure 3A displays the polynomial regression for total LNC considering different nitrogen levels associated with A. brasilense inoculation and biostimulant application, regardless of the growing season. In uninoculated treatments, total LNC increased linearly with rising N fertilizer levels when no biostimulant was applied, with an estimated increase of about 0.05 g kg-1 per kg N ha-1 applied. In uninoculated treatments with biostimulant, an increase in N fertilizer levels followed a quadratic model, where the maximum LNC (30.10 g kg-1) was estimated with the application of 94 kg N ha-1. In treatments inoculated with different N levels, but without biostimulant, the highest LNC (29.90 g kg-1) would theoretically be reached with 114 kg N ha-1. In inoculated treatments with biostimulant, total LNC increased linearly with higher N fertilizer levels, with an estimated increase of about 0.06 g kg-1 per kg N ha-1 applied (Figure 3A).
Total leaf nitrogen content as a function of N fertilizer levels at inoculation (inoculated and uninoculated), associated with biostimulant application [without biostimulant (A.B) and with biostimulant (P.B)] in different growing seasons (A) and total leaf nitrogen content as a function of nitrogen fertilizer levels in association with the growing season, Azospirillum brasilense seed inoculation level, and biostimulant application (B). Summer, Maringá, Paraná State, Brazil.
Further analysis of the interaction (N x GS) for total LNC revealed distinct patterns that conformed to linear increases (2017/2018 and 2019/2020) and quadratic models (2018/2019), regardless of A. brasilense inoculation and biostimulant application (Figure 3B). In 2017/2018, the estimated increase, as indicated by the angular coefficient, was 0.07 g kg-1 per kg N ha-1 applied to the soil. In 2018/2019, the maximum LNC estimated by the quadratic model was 28.10 g kg-1 with 103 kg N ha-1. In 2019/2020, the increase in total LNC estimated by the angular coefficient was approximately 0.05 g kg-1 per kg N ha-1 added. These findings align with the results of previous studies by Galindo et al. (2019) and Pelloso et al. (2023), which also reported that leaf N content increased with higher N fertilizer doses in corn crops.
As per Fei, Crouse, Papadopoulos, and Vessey (2019), while the mechanisms governing actions of beneficial soil microorganisms and biostimulant effects on plant growth remain incompletely understood, they operate through modes of action that enhance nutrient uptake by plants. Consequently, the results depicted in Figure 3A can be attributed to an improved root system development and increased A. brasilense population in corn plant rhizosphere, resulting in enhanced nutrient absorption (Fukami et al., 2018).
Nitrogen absorption and cytokine concentration appear to be intricately linked. Nitrogen supply triggers an increase in cytokine concentration and transport within plants, indicating that this plant hormone communicates long-distance N availability in roots and shoots (De-Bang et al., 2021). Nevertheless, plant responses to N supply are not solely determined by their interaction with cytokines; they are also influenced by a complex signaling network involving other classes of hormones. For example, in rice crops, Xu et al. (2015) observed that changes in the shoots in response to nitrogen additions are controlled by the interplay between cytokines, auxins, and strigolactones.
Table 3 illustrates that, in the absence of N fertilizer, total LNC in 2019/2020 was higher than in 2017/2018 (p < 0.05). Applications of 60 and 120 kg N ha-1 promoted the highest LNC in the 2017/2018 and 2019/2020 growing seasons, differing statistically from 2018/2019 (p < 0.05). This can be attributed to a drought stress experienced during the second year of the crop. Notably, leaf N concentration fell within the suitable range (27 to 35 g kg-1) (Cantarella et al., 1997), except for plants without N fertilizer and at a dose of 30 kg N ha-1 (Table 3).
Table 4 indicates that during the 2018/2019 growing season, the biostimulant had a significant impact (p < 0.05) on dehusked ear yield (DEY), irrespective of N levels and A. brasilense inoculation. Moreover, the use of biostimulant increased DEY by 0.55 Mg ha-1 (5.08%) compared to treatments without it.
The amount of nutrients supplied through mass flow depends on transpiration and their concentration in soil solution. When both factors are high, mass flow plays a significant role in acquiring nitrogen ions (Plett et al., 2020). Our findings suggest that N fertilization, whether alone or in conjunction with A. brasilense and/or biostimulant, during summer droughts in subtropical regions can mitigate a transpiration reduction. This, in turn, reduces photosynthetic carbon assimilation and leads to increased absorption of mobile nutrients, such as ammonium (NH4 +) and nitrate (NO3 -) in soil solution, increasing total LNC.
However, A. brasilense seed inoculation did not significantly increase (p > 0.05) total LNC for the studied N levels and biostimulant doses. Likewise, leaf application of biostimulant did not significantly raise total LNC for the analyzed N and seed inoculation levels.
Higher leaf nitrogen content contributes to increased plant growth and development, which leads to greater carbohydrate accumulation through photosynthesis. Consequently, corn plants enhance their ability to allocate carbohydrates to the roots, enabling them to use available nitrogen, whether from soil or fertilizer, more efficiently (Meira et al., 2009).
The lack of significant responses in the productivity of the 2017/2018 and 2019/2020 growing seasons, both in plants treated and untreated with the biostimulant, could be attributed to favorable climate conditions, particularly higher rainfall, during those crop cycles. These conditions naturally supported higher yields even without the application of the biostimulant. In contrast, during the 2018/2019 growing season, despite total rainfall being within the suitable range for the crop (Figure 1), irregular water distribution and dry spells between stages V12 and V15 and VT and R1 led to drought stress, negatively affecting corn yield. Thus, applying the biostimulant may have provided beneficial effects on the plants during these critical phenological phases.
The increase in the weight of green corn in environments with limited water availability can also be attributed to the presence of nutrients, plant growth regulators like cytokinins, and osmolytes such as glycine betaine in the biostimulant composition. These components support plant nutrition and contribute to higher DEY.
Similarly, a study by Shemi et al. (2021) observed that the exogenous application of glycine betaine and zinc mitigated the adverse effects of water deficit in corn. It increased antioxidant enzymes, the accumulation of soluble sugars, and free proline while reducing reactive oxygen species and lipoperoxidation rates. Foliar glycine betaine application also improved the net photosynthetic rate, stomatal conductance, transpiration rate, stabilized photosynthetic pigments, and increased relative leaf water content. These mechanisms are crucial for ensuring ear production in green corn crops under drought stress conditions.
Regardless of A. brasilense inoculation, biostimulant application, and the growing season, the increases in dehusked ear yield estimated by the angular coefficients were 0.0316 Mg ha-1 in 2017/2018, 0.0126 Mg ha-1 in 2018/2019, and 0.0081 Mg ha-1 per kg N ha-1 applied in 2019/2020 (Figure 4A). For commercial ear yield (CEY), regardless of seed inoculation, biostimulant application, and growing season, the increase estimated by the angular coefficient was approximately 0.016 Mg ha-1 per kg N ha-1 applied (Figure 4B).
Dehusked ear yield as a function of nitrogen fertilizer levels in association with growing season, at an average Azospirillum brasilense seed inoculation level, biostimulant application (A); and commercial ear yield as a function of nitrogen fertilizer levels, at an average Azospirillum brasilense seed inoculation level, biostimulant application, and growing seasons (B). Summer, Maringá, Paraná State, Brazil.
Our findings align with those of Galindo et al. (2019), demonstrating that increasing N levels can improve corn yields. According to these authors, N is readily available in the soil solution when the plants require larger quantities of this nutrient. This suggests that the N applied as base dressing might already be present in the soil solution, and when additional N is supplied, roots have access to a greater quantity of the element for absorption.
The superior dehusked and commercial ear yield results obtained during the 2017/2018 growing season in comparison to those recorded in 2018/2019 and 2019/2020 (p < 0.05) (Table 5) indicate that a combination of favorable climate conditions (Figure 1) and soil fertility played a role in promoting green corn ear production during the first year of cultivation.
Dehusked ear yield (DEY) at an average Azospirillum brasilense seed inoculation level and biostimulation application during the growing seasons at each N fertilizer level; commercial ear yield (CEY) as a function of growing season, at an average nitrogen fertilizer level, seed inoculation, and biostimulant application. Summer, Maringá, Paraná State, Brazil.
The environmental conditions experienced during the 2019/2020 growing season led to poor plant development, characterized by shorter plants and a reduced LAI compared to the two previous crop cycles. While yield depends on net photosynthesis, especially after flowering, leaf area plays a crucial role in the development of organs like the ears (Subedi & Ma, 2005). Thus, plant growth and LAI have a direct impact on ear yield (Okumura et al., 2014).
The protein content of the kernels (PROT) responded significantly to the second-order interaction among N levels, seed inoculation, and growing seasons (N x I x GS). In uninoculated treatments during the 2017/2018 growing season, regardless of biostimulant application, the increase in PROT estimated by the angular coefficient was approximately 0.015% per kg N ha-1 applied. In inoculated treatments during the same season, the maximum protein content (10.80%) was estimated with 71 kg N ha-1. In the 2018/2019 season, the maximum kernel protein content of 10.50% and 10.40% was obtained with 84 and 115 kg N ha-1 in the uninoculated and inoculated treatments, respectively. In the 2019/2020 growing season, the maximum kernel protein content was 11.65% in the uninoculated treatments and 11.30% in the inoculated treatments, achieved with 79 and 88 kg N ha-1, respectively (Figure 5A).
Regardless of A. brasilense inoculation and growing seasons, there was a noticeable difference between response curves for N fertilization associated with biostimulant application, with a linear fit for treatments without biostimulant and quadratic for those with it (Figure 5B). Without biostimulant, PROT increase estimated by angular coefficient was about 0.017% per kg N ha-1 added. With biostimulant, the highest PROT (10.80%) would theoretically be reached with 77 kg N ha-1.
Raw kernel protein content as a function of nitrogen fertilizer level in inoculated and uninoculated treatments, during the 2017/2018, 2018/2019 and 2019/2020 summer crop seasons, at an average biostimulation application (A); raw kernel protein content as a function of nitrogen fertilizer level associated with biostimulant application, without biostimulant (A.B) and with biostimulant (P.B), at an average Azospirillum brasilense seed inoculation level, and growing seasons (B). Summer, Maringá, Paraná State, Brazil.
Regarding the average PROT under different nitrogen fertilization levels combined with seed inoculation, there was a significant decrease of 11.05% (from 11.49 to 10.22%) in kernel protein content only when 120 kg N ha-1 was used in 2017/2018, and a decrease of 12.32% (from 12.50 to 10.96%) when 90 kg N ha-1 was used in 2019/2020. The other nitrogen fertilizer levels, regardless of seed inoculation, had no significant effect. While the factors that influence plant responses to A. brasilense in the field are not completely understood, previous reports have suggested that Azospirillum bacteria perform better in sandy soils (Ferreira et al., 2013). Thus, the high clay content (520 g kg-1) in the soil in this study may have counteracted benefits from A. brasilense seed inoculation.
Organic matter content and soil pH also play a role in the composition of root exudates, which can affect interactions between plants and bacteria (Tadra-Sfeir et al., 2011; Martins et al., 2018). Based on our results, applying higher N levels in combination with A. brasilense inoculation had a negative impact on kernel protein content, suggesting that N levels above 90 kg N ha-1 may offset A. brasilense inoculation benefits in terms of kernel quality.
The 2017/2018 and 2019/2020 growing seasons provided better conditions for accumulating protein in green corn kernels (Table 6). This can be attributed to higher rainfall levels during these seasons, as protein accumulation in kernel endosperm is susceptible to drought stress (Ayub et al., 2021). Reduced protein content under drought stress may result from a significant decrease in photosynthetic rates or decrease in availability of protein assimilates, which are essential for protein synthesis (Ayub et al., 2021).
Table 7 indicates that applying 60 kg N ha-1 increased leaf protein content by 0.61%. However, with an application of 90 kg N ha-1 and biostimulant use, there was a decrease in kernel protein content by 0.69%.
Average raw kernel protein content (%) as a function of biostimulant management, without biostimulant (A.B) and with biostimulant (P.B), at the following N levels: 0, 30, 60, 90, and 120 kg ha-1, at an average Azospirillum brasilense seed inoculation level and different growing seasons. Summer, Maringá, Paraná State, Brazil.
The increase in PROT with the use of biostimulant at 60 kg N ha-1 might have been due to the protective effect of quaternary NH4 + compounds, such as glycine betaine. These compounds can prevent enzyme, complex protein degradation, and provide stability to membranes as a response to several types of environmental stress, resulting in an increase in the photosynthetic rate, which, in turn, can improve kernel protein content (Layek et al., 2015). However, this effect likely did not occur at the higher fertilization levels tested, such as 90 kg N ha-1, due to the lower production of protein amino acids and other metabolites, which remained stored in plant tissues.
Our findings support those reported by Carillo et al. (2019) and highlight that leaf application of biostimulants, under excess N conditions, can decrease the nutritional quality of certain crops, as a consequence of a decline in essential amino acid content, potentially leading to increased accumulation of antinutrients such as NO3 -. However, it is important to note that information on the effect of interactions between N levels and biostimulant application on corn kernel protein content is limited in the literature, and the few existing reports were conducted with different vegetable crops, not green corn.
Conclusion
Increasing nitrogen fertilizer levels have a positive impact on morphological traits, leaf nitrogen concentration, commercial corn production, and kernel protein content. Azospirillum brasilense seed inoculation does not promote significant increases in ear yield or kernel protein content. In fact, when inoculation is combined with the two highest nitrogen levels (90 and 120 kg N ha-1), there is a decline in kernel protein content. Leaf application of biostimulant increased ear weight during the 2018/2019 growing season, which also led to higher protein content. The study suggests that A. brasilense seed inoculation and exogenous biostimulant application do not reduce the need for nitrogen fertilizer in green corn production.
Acknowledgements
We would like to thank the National Council for Scientific and Technological Development (CNPq) for its financial support and the State University of Maringá for supporting the research
References
-
Albuquerque, C. J. B., Von Pinho, R. G., Borges, I. D., Souza Filho, A. X., & Fiorini, I. V. A. (2008). Desempenho de híbridos experimentais e comerciais de milho para produção de milho verde. Ciência e Agrotecnologia, 32(3), 768-775. DOI: https://doi.org/10.1590/S1413-70542008000300010
» https://doi.org/https://doi.org/10.1590/S1413-70542008000300010 -
Ayub, M., Ashraf, M. Y., Kausar, A., Saleem, S., Anwar, S., Altay, V., & Ozturk, M. (2021). Growth and physio-biochemical responses of maize (Zea mays L.) to drought and heat stresses. Plant Biosystems, 155(3), 535-542. DOI: https://doi.org/10.1080/11263504.2020.1762785
» https://doi.org/https://doi.org/10.1080/11263504.2020.1762785 - Banzatto, D. A., & Kronka, S. N. (2006). Experimentação agrícola (4. ed.). Jaboticabal, SP: Funep.
- Brasil. (2005). Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA). Métodos físico-químicos para análise de alimentos (Série A: Normas Técnicas e Manuais Técnicos, cap. IV, p. 116-141). Brasília, DF: Ministério da Saúde.
- Cantarella, H., van Raij, B., & Camargo, C. E. O. (1997). Cereais. In B. van Raij, H. Cantarella, J. A. Quaggio, & A. M. C. Furlani (Org.), Recomendações de adubação e calagem para o estado de São Paulo (Boletim Técnico, 100, p. 45-71). Campinas, SP: IAC.
-
Carillo, P., Colla, G., Fusco, G. M., Dell’aversana, E., El-Nakhel, C., Giordano, M., ... Rouphael, Y. (2019). Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy, 9(8), 1-22. DOI: https://doi.org/10.3390/agronomy9080450
» https://doi.org/https://doi.org/10.3390/agronomy9080450 -
De-Bang, T. C., Husted, S., Laursen, K. H., Persson, D. P., & Schjoerring, J. K. (2021). The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytologist, 229(5), 2446-2469. DOI: https://doi.org/10.1111/nph.17074
» https://doi.org/https://doi.org/10.1111/nph.17074 -
Fei, H., Crouse, M., Papadopoulos, Y. A., & Vessey, J. K. (2019). Improving biomass yield of giant Miscanthus by application of beneficial soil microbes and a plant biostimulant. Canadian Journal of Plant Science, 100(1), 29-39. DOI: https://doi.org/10.1139/cjps-2019-0012
» https://doi.org/https://doi.org/10.1139/cjps-2019-0012 -
Ferreira, A. S., Pires, R. R., Rabelo, P. G., Oliveira, R. C., Luz, J. M. Q., & Brito, C. H. (2013). Implications of Azospirillum brasilense inoculation and nutrient addition on maize in soils of the Brazilian Cerrado under greenhouse and field conditions. Applied Soil Ecology, 72, 103-108. DOI: https://doi.org/10.1016/j.apsoil.2013.05.020
» https://doi.org/https://doi.org/10.1016/j.apsoil.2013.05.020 -
Ferreira, D. F. (2014). Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia , 38(2), 109-112. DOI: https://doi.org/10.1590/S1413-70542014000200001
» https://doi.org/https://doi.org/10.1590/S1413-70542014000200001 -
Food and Agricuture Organization of the United Nations [FAO]. (2023). Faostat: production crops (maize) Retrieved on Mar. 10, 2023 from 10, 2023 from http://www.fao.org/faostat/en/#data
» http://www.fao.org/faostat/en/#data -
Francis, C. A., Rutger, J. N., & Palmer, A. F. E. (1969). A rapid method for plant leaf area estimation in maize (Zea mays L.). Crop Science, 9(5), 537-539. DOI: https://doi.org/10.2135/cropsci1969.0011183X000900050005x
» https://doi.org/https://doi.org/10.2135/cropsci1969.0011183X000900050005x -
Fukami, J., Cerezini, P., & Hungria, M. (2018). Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express, 8(73), 1-12. DOI: https://doi.org/10.1186/s13568-018-0608-1
» https://doi.org/https://doi.org/10.1186/s13568-018-0608-1 -
Galindo, F. S., Teixeira Filho, M. C. M., Buzetti, S., Pagliari, P. H., Santini, J. M. K., Alves, C. J., … Arf, O. (2019). Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense Agronomy Journal, 111(4), 1985-1997. DOI: https://doi.org/10.2134/agronj2018.07.0481
» https://doi.org/https://doi.org/10.2134/agronj2018.07.0481 - Instituto Agronômico do Paraná [IAPAR]. (1994). Cartas climáticas básicas do Estado do Paraná Londrina, PR: IAPAR.
-
Layek, J., Das, A., Ramkrushna, G. I., Trivedi, K., Yesuraj, D., Chandramohan, M., … Ghosh, A. (2015). Seaweed sap: a sustainable way to improve productivity of maize in North-East India. International Journal of Environmental Studies, 72(2), 305-315. DOI: https://doi.org/10.1080/00207233.2015.1010855
» https://doi.org/https://doi.org/10.1080/00207233.2015.1010855 -
Liu, G., Hou, P., Xie, R., Ming, B., Wang, K., Liu, W., … Li, S. (2019). Nitrogen uptake and response to radiation distribution in the canopy of high‐yield maize. Crop Science , 59(3), 1236-1247. DOI: https://doi.org/10.2135/cropsci2018.09.0567
» https://doi.org/https://doi.org/10.2135/cropsci2018.09.0567 -
Martins, M. R., Jantalia, C. P., Reis, V. M., Döwich, I., Polidoro, J. C., Alves, B. J. R., ... Urquiaga, S. (2018). Impact of plant growth-promoting bacteria on grain yield, protein content, and urea-15 N recovery by maize in a Cerrado Oxisol. Plant and Soil, 422(1-2), 239-250. DOI: https://doi.org/10.1007/s11104-017-3193-1
» https://doi.org/https://doi.org/10.1007/s11104-017-3193-1 -
Meira, F. A., Buzetti, S., Andreotti, M., Arf, O., Sá, M. E., & Andrade, J. A. C. (2009). Fontes e épocas de aplicação do nitrogênio na cultura do milho irrigado. Semina: Ciências Agrárias, 30(2), 275-284. DOI: http://dx.doi.org/10.5433/1679-0359.2009v30n2p275
» https://doi.org/http://dx.doi.org/10.5433/1679-0359.2009v30n2p275 -
Nemeskéri, E., Molnár, K., Rácz, C., Dobos, A. C., & Helyes, L. (2019). Effect of water supply on spectral traits and their relationship with the productivity of sweet corns. Agronomy, 9(2), 1-17. DOI: https://doi.org/10.3390/agronomy9020063
» https://doi.org/https://doi.org/10.3390/agronomy9020063 -
Ngumbi, E., & Kloepper, J. (2016). Bacterial-mediated drought tolerance: current and future prospects. Applied Soil Ecology , 105, 109-125. DOI: https://doi.org/10.1016/j.apsoil.2016.04.009
» https://doi.org/https://doi.org/10.1016/j.apsoil.2016.04.009 -
Okumura, R. S., Vidigal Filho, P. S., Scapim, C. A., Marques, O. J., Franco, A. A. N., Souza, R. S., & Reche, D. L. (2014). Effects of nitrogen rates and timing of nitrogen topdressing applications on the nutritional and agronomic traits of sweet corn. Journal of Food, Agriculture & Environment, 12(2), 391-398. DOI: https://doi.org/10.1234/4.2014.5164
» https://doi.org/https://doi.org/10.1234/4.2014.5164 -
Pelloso, M. F., Vidigal Filho, P. S., Scapim, C. A., Ortiz, A. H. T., Numoto, A. Y., & Freitas, I. R. M. (2023). Agronomic performance and quality of baby corn in response to the inoculation of seeds with Azospirillum brasilense and nitrogen fertilization in the summer harvest. Heliyon, 9(4), 1-14. DOI: https://10.1016/j.heliyon.2023.e14618
» https://doi.org/https://10.1016/j.heliyon.2023.e14618 -
Plett, D. C., Ranathunge, K., Melino, V. J., Kuya, N., Uga, Y., & Kronzucker, H. J. (2020). The intersection of nitrogen nutrition and water use in plants: new paths toward improved crop productivity. Journal of Experimental Botany, 71(15), 4452-4468. DOI: https://doi.org/10.1093/jxb/eraa049
» https://doi.org/https://doi.org/10.1093/jxb/eraa049 -
Posch, B. C., Kariyawasam, B. C., Bramley, H., Coast, O., Richards, R. A., Reynolds, M. P., … Atkin, O. K. (2019). Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. Journal of Experimental Botany , 70(19), 5051-5069. DOI: https://doi.org/10.1093/jxb/erz257
» https://doi.org/https://doi.org/10.1093/jxb/erz257 - Ritchie, S. W., Hanway, J. J., & Benson, G. O. (1993). How a corn plant develops (Special Report, 48). Ames, US: Iowa State University of Science and Technology.
- Santos, H. G., Jacomine, P. K. T., Anjos, L. H. C., Oliveira, V. A., Lumbreras, J. F., Coelho, M. R., ... Cunha, T. J. F. (2018). Sistema brasileiro de classificação de solos (5. ed.). Brasília, DF: Embrapa.
-
Shemi, R., Wang, R., Gheith, E. S. M., Hussain, H. A., Hussain, S., Irfan, M., … Wang, L. (2021). Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Scientific Reports, 11(3195), 1-14. DOI: https://doi.org/10.1038/s41598-021-82264-7
» https://doi.org/https://doi.org/10.1038/s41598-021-82264-7 -
Singh, I., Anand, K. V., Solomon, S., Shukla, S. K., Rai, R., Zodape, S. T., & Ghosh, A. (2018). Can we not mitigate climate change using seaweed based biostimulant: A case study with sugarcane cultivation in India. Journal of Cleaner Production, 204, 992-1003. DOI: https://doi.org/10.1016/j.jclepro.2018.09.070
» https://doi.org/https://doi.org/10.1016/j.jclepro.2018.09.070 -
Subedi, K. D., & Ma, B. L. (2005). Ear position, leaf area, and contribution of individual leaves to grain yield in conventional and leafy maize hybrids. Crop Science , 45(6), 2246-2257. DOI: https://doi.org/10.2135/cropsci2004.0653
» https://doi.org/https://doi.org/10.2135/cropsci2004.0653 -
Tadra-Sfeir, M. Z., Souza, E. M., Faoro, H., Müller-Santos, M., Baura, V. A., Tuleski, T. R., ... Monteiro, R. A. (2011). Naringenin regulates expression of genes involved in cell wall synthesis in Herbaspirillum seropedicae Applied and Environmental Microbiology, 77(6), 2180-2183. DOI: https://doi.org/10.1128/AEM.02071-10
» https://doi.org/https://doi.org/10.1128/AEM.02071-10 -
Tei, F., De Neve, S., de Haan, J., & Kristensen, H. L. (2020). Nitrogen management of vegetable crops. Agricultural Water Management, 240, 1-13. DOI: https://doi.org/10.1016/j.agwat.2020.106316
» https://doi.org/https://doi.org/10.1016/j.agwat.2020.106316 -
Xu, G., Fan, X., & Miller, A. J. (2012). Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology, 63, 153-182. DOI: https://doi.org/10.1146/annurev-arplant-042811-105532
» https://doi.org/https://doi.org/10.1146/annurev-arplant-042811-105532 -
Xu, J., Zha, M., Li, Y., Ding, Y., Chen, L., Ding, C., & Wang, S. (2015). The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Reports, 34(9), 1647-1662. DOI: https://doi.org/10.1007/s00299-015-1815-8
» https://doi.org/https://doi.org/10.1007/s00299-015-1815-8
Publication Dates
-
Publication in this collection
17 Mar 2025 -
Date of issue
2025
History
-
Received
07 Sept 2023 -
Accepted
11 Dec 2023

 Optimizing nitrogen fertilization with Azospirillum brasilense and biostimulants for green corn
Optimizing nitrogen fertilization with Azospirillum brasilense and biostimulants for green corn