Abstract
Phosphogypsum (PG), a byproduct of phosphoric acid production, shows potential as a substitute for traditional gypsum in cement formulations. However, the fluorides and phosphates in its composition can delay setting time and reduce early strength in Portland cement. The use of hydration and strength accelerators can counteract these effects, but the literature lacks sufficient information on the subject. This research evaluates the influence of alternative accelerators on the hydration of cement with phosphogypsum. Sodium chloride (NaOH), calcium chloride (CaCl2), sodium hydroxide (NaCl), and sodium silicate (Na2SiO3) were analyzed. Isothermal calorimetry and compressive strength tests were conducted on cement pastes. NaOH showed the highest 24-hour compressive strength and cumulative heat, suggesting its potential as a strength accelerator. Na2SiO3 exhibited the lowest performance compared to the reference sample (REF). The strengths and reaction rates of CaCl2 were similar to those of the REF. NaCl displayed higher strengths and cumulative heat than the REF, indicating its effectiveness as an accelerator.
Keywords
Phosphogypsum; Alternative accelerators; Hydration
Resumo
O fosfogesso (FOS), um subproduto da produção de ácido fosfórico, mostra potencial como substituto do gesso tradicional em formulações de cimento. No entanto, o flúor e fósforo presente na sua composição podem atrasar o tempo de pega e reduzir a resistência inicial no cimento Portland. O uso de aceleradores para hidratação e aumento de resistência pode contrabalançar esses efeitos, mas a literatura carece de informações suficientes. Esta pesquisa avalia a influência de aceleradores alternativos na hidratação do cimento com fosfogesso. Cloreto de sódio (NaOH), cloreto de cálcio (CaCl2), hidróxido de sódio (NaCl) e silicato de sódio (Na2SiO3) foram analisados. Calorimetria isotérmica e testes de resistência à compressão foram realizados em pastas de cimento. NaOH apresentou a maior resistência à compressão em 24 horas e calor acumulado, sugerindo seu potencial como acelerador de resistência. Na2SiO3 mostrou o desempenho mais baixo em comparação com a amostra de referência (REF). As resistências e taxas de reação do CaCl2 foram semelhantes às da REF. NaCl exibiu resistências e calor acumulado superiores aos de REF, indicando sua eficácia como acelerador.
Palavras-chave
Fosfogesso; Aceleradores alternativos; Hidratação
Introduction
Phosphogypsum (PG) is a byproduct of the phosphate fertilizer industry generated during phosphoric acid production. PG accumulates in piles across 52 countries, reaching approximately 3-4 billion tons with an annual increase of about 200 million tons (Hermann; Kraus; Hermann, 2018). PG poses significant environmental challenges, often stored in tanks or disposed of in the ocean (Akfas et al., 2024; Radhouan El Zrelli et al., 2019). Major accumulators include China, Brazil, Spain, and the United States (Cánovas et al., 2017; Cao et al., 2022a; Qin et al., 2023). However, of the abovementioned amount, only approximately 15% are recycled, such as in construction materials or agriculture (Tayibi et al., 2009). Effective management and integration of this waste into the circular economy are crucial scientific concerns.
In Portland cement production, due to the chemical and mineralogical composition of phosphogypsum (PG) being similar to natural gypsum, it acts in the regulation of the hydration of tricalcium aluminate (3CaO·Al2O3, or C3A in the cement’s chemical nomenclature), which is the most reactive phase of Portland clinker (Kirchheim et al., 2010; Mehta; Monteiro, 2014). By incorporating calcium sulfate in the cement grinding, either in the form of natural gypsum or phosphogypsum, the objective is that during the reaction of C3A, an insoluble intermediate phase, ettringite, is formed, delaying the hydration of C3A.
Thus, PG emerges as an alternative where natural gypsum reserves are scarce (Costa et al., 2022; Cui et al., 2023; Haneklaus et al., 2022; Maazoun; Bouassida, 2020; Qamouche et al., 2020). In Brazil, where 93% of natural gypsum sources are concentrated in the Northeast, cement plants in the Southeast and South face logistical challenges transporting materials over 4,000 kilometers (Canut et al., 2008; Costa et al., 2022).
However, PG may contain minor levels of phosphorus (P2O5) and fluorine (F-) (0.1% to 1.8%), heavy metals (Cr and Co), and radionuclides (Cánovas et al., 2018; Ennaciri; Bettach, 2024; Ramteke et al., 2018). In cement use, impurities like fluorides (F-) and phosphates (P2O5) delay setting times and reduce initial mechanical strength (Costa et al., 2021, 2022).
One approach to mitigate these challenges involves incorporating setting and strength-accelerating admixtures. In this context, this study aims to evaluate the effect of using NaCl, CaCl2, NaOH, and Na2SiO3 as potential hydration enhancers for Portland cement containing phosphogypsum (PG) to mitigate setting delays and reduce initial strength loss. Thus, this assessment was carried out through an experimental program that studied the heat of hydration and the initial strengths of the mentioned cementitious systems. The main contribution of this study lies in bridging the current gap concerning the integration of PG into cement with alternative accelerators. This area remains relatively underexplored in the existing literature. Additionally, this study helps to promote a paradigm shift in the perception of PG from waste to valuable material.
Theoretical framework
Soluble and moderately soluble F- and P2O5 have been appointed in the literature as the primary contributors to delayed setting times and reduced initial mechanical strength in cement containing PG (Cao et al., 2022b, 2024; Zhang et al., 2022). Some researchers argue that the delay in cement reaction due to PG is attributed to the precipitation of calcium phosphate compounds (such as hydroxyapatite - Ca₁₀(PO₄)₆(OH)₂) and calcium fluoride (CaF2), which hinder the dissolution and hydration of cement (Bénard et al., 2005, 2008; Tabikn; Miller, 1971). Studies also highlight the soluble phosphorus forms present in PG (e.g., H3PO4, H2PO4-, and HPO42−) as critical factors influencing the reaction delay with binders or as a binder (Jia; Wang; Luo, 2021; Zhang et al., 2022). Holanda, Schmidt, and Quarcioni (2017) suggested that P2O5 concentrations between 0.83% and 1.64% in PG cause the most significant delays in the initial hydration phase in cement. Furthermore, incorporating water-reducing admixtures adds another layer of complexity to the behavior of the cementitious system when combined with PG, further delaying the reaction (Andrade Neto; De La Torre; Kirchheim, 2021a; Holanda; Schmidt; Quarcioni, 2017; Qi et al., 2022).
Therefore, using accelerators could offer a viable solution to address this issue. Among various accelerators, water-soluble inorganic salts are notable for enhancing initial strength performance by accelerating the hydration of tricalcium silicate (C3S) (Ramachandran, 1996). The influence of different anions and cations on this hydration acceleration follows a specific order under similar molarity conditions, as reported (Dorn; Blask; Stephan, 2022; Taylor, 1990):
Anion: Br- ≈ Cl- > SCN- > I- > NO3- > ClO4-
Cation: Ca2+ > Ni2+ > Ba2+ > Mg2+ > Fe3+ > Cr2+ > Co2+ > La3+ ≫ NH4+, K+ > Li+ > Na+
While the accelerating effect of these inorganic salts is recognized, and there are theories about their mechanisms of action in Portland cement, the mode of operation is not widely understood.
On the other hand, common organic compounds used in the production of Portland cement as grinding aid and hydration enhancers include alkanolamines, such as triethanolamine (TEA), triisopropanolamine (TIPA), and diethylethanolamine (DEAE) (Derakhshani; Ghadi; Ebrahim Vahdat, 2023; Lu et al., 2020). Glycols, such as propylene glycol (PG), monoethylene glycol (MEG), and diethylene glycol (DEG), also fall into this category. Alkanolamines influence cement hydration and improve grinding (Xie et al., 2023; Xu et al., 2017).
However, the hydration enhancers can have high-cost, toxicity, and some types may be subject to legal acquisition restrictions (military and federal police). Therefore, in balancing costs and input availability, alternative accelerators such as sodium chloride (NaCl), calcium chloride (CaCl2), sodium hydroxide (NaOH), and sodium silicate (Na2SiO3) emerge as promising alternatives to be tested.
Historically, calcium chloride (CaCl2) has been the most widely used inorganic salt setting accelerator, affecting the hydration of C3S, C3A, and gypsum (Ramachandran, 1996). Cheung et al. (2011) reported that the increase in the C-S-H porosity due to the presence of CaCl2 leads to the formation of additional nucleation points, thereby accelerating C-S-H growth during C3S hydration. In contrast, Wang, Chen, and Wang (2016) suggested that CaCl2 hinders the ettringite formation in cement mixtures exposed to low temperatures, a common condition encountered in cement used in oil wells.
Sodium chloride (NaCl) is also known to accelerate the reaction of Portland cement at specific dosages. Rodrigues Lago et al. (2017), when evaluating the influence of salts on the hydration of cement for oil wells, showed that lower NaCl contents (up to 10% by mass of water) would accelerate hydration reactions, while dosages above 20% would delay the C3S and C3A reactions. Another study analyzing NaCl solutions in the hydration of cement pastes suggested that chloride ions accelerate hydration reactions during nucleation and crystal growth (Li et al., 2021). However, Zhu et al. (2023) emphasized that the influence of chloride salts is highly dependent on the type of cation to which they are associated.
Regarding NaOH, studies in the presence of C3S and C2A indicated an acceleration of the hydration of these phases and higher heat release during hydration when hydrated with NaOH (Anchez-Herrero; Fernandez-Jim Enez; Palomo, 2015). Similarly, Mota, Matschei, and Scrivener (2018) identified the acceleration of hydration and gain in strength in cementitious systems with NaOH in the early hours of the reaction.
Sodium silicate (Na2SiO3) is also known in the literature for its use as a base for accelerators (alkaline silicates) and as an activator in cementitious systems with slag, fly ash, and in the production of geopolymers (Hosein; Mousavinejad; Sammak, 2022; Wang et al., 2021; Yang et al., 2023). The alkaline system produced by this compound and the soluble silicates promotes setting acceleration through the precipitation of calcium silicates (Prudhcio Junior, 1998; Ramachandran, 1996).
Method
Experimental research was conducted and divided into two stages. The first stage involved the physicochemical characterization and properties of the cement assessed in the fresh and hardened states without the potential accelerators (PA). On the other hand, the second stage focused on evaluating pastes of this cement in the presence of PA by measuring the released reaction heat and the gain in strength at early ages. In this study, Portland cement (CP V-ARI RS) was evaluated and produced for research purposes with 100% phosphogypsum (PG) as a source of calcium sulfate to regulate the cement setting, combined with PA. The cement was comprised of clinker and phosphogypsum following C563-20 (ASTM, 2020) and complied with the 4.5 % SO3 limit requested by NBR 16697 (ABNT, 2018a). A cement partner company supplied the cement. Information regarding the chemical composition (XRF) of the PG used in this study is presented in Table 1. The data indicate a P2O5 content of approximately 1% and an F- content of 0.16%, which could retard cement hydration. The SO3 content is 38%, similar to other calcium sulfate sources such as natural gypsum.
The materials tested as accelerators were analytical-grade sodium chloride (NaCl), calcium chloride (CaCl2), sodium hydroxide (NaOH), and sodium silicate (Na2SiO3). The dosages used for each accelerator are described in Table 2. These dosages were adopted based on recommendations from the literature (Dorn; Blask; Stephan, 2022; Mota; Matschei; Scrivener, 2018; Wang et al., 2022). Chloride-based materials were dosed considering the maximum limits of Cl- ions established by NBR 12655 (ABNT, 2015a).
Physicochemical characterization and properties of cement without PA (Potential accelerators)
The physicochemical characterization, provided by the cement partner company, is presented in Table 3, including: Initial Setting Time (IS) in minutes; Final Setting Time (FS) in minutes, NBR 16607 (ABNT, 2018b); A (%) - Water required for normal consistency paste, NBR 16606 (ABNT, 2018c); Loss on Ignition (%), NBR 17086-6 (ABNT, 2023); #200 - percentage retained on the 75 μm sieve, NBR 11579 (ABNT, 2012); Blaine - Fineness determination by the Air Permeability Method, NBR 16372 (ABNT, 2015b); R1, R7 e R28 (MPa) – Compressive strength in the mortar at ages 01, 07, and 28 days, NBR 7215 (ABNT, 2019). A lime-saturated solution was used to cure the samples. These results are also presented in Vieira Alves (2021).
Heat of hydration and strength of cement pastes with PA (Potential accelerators)
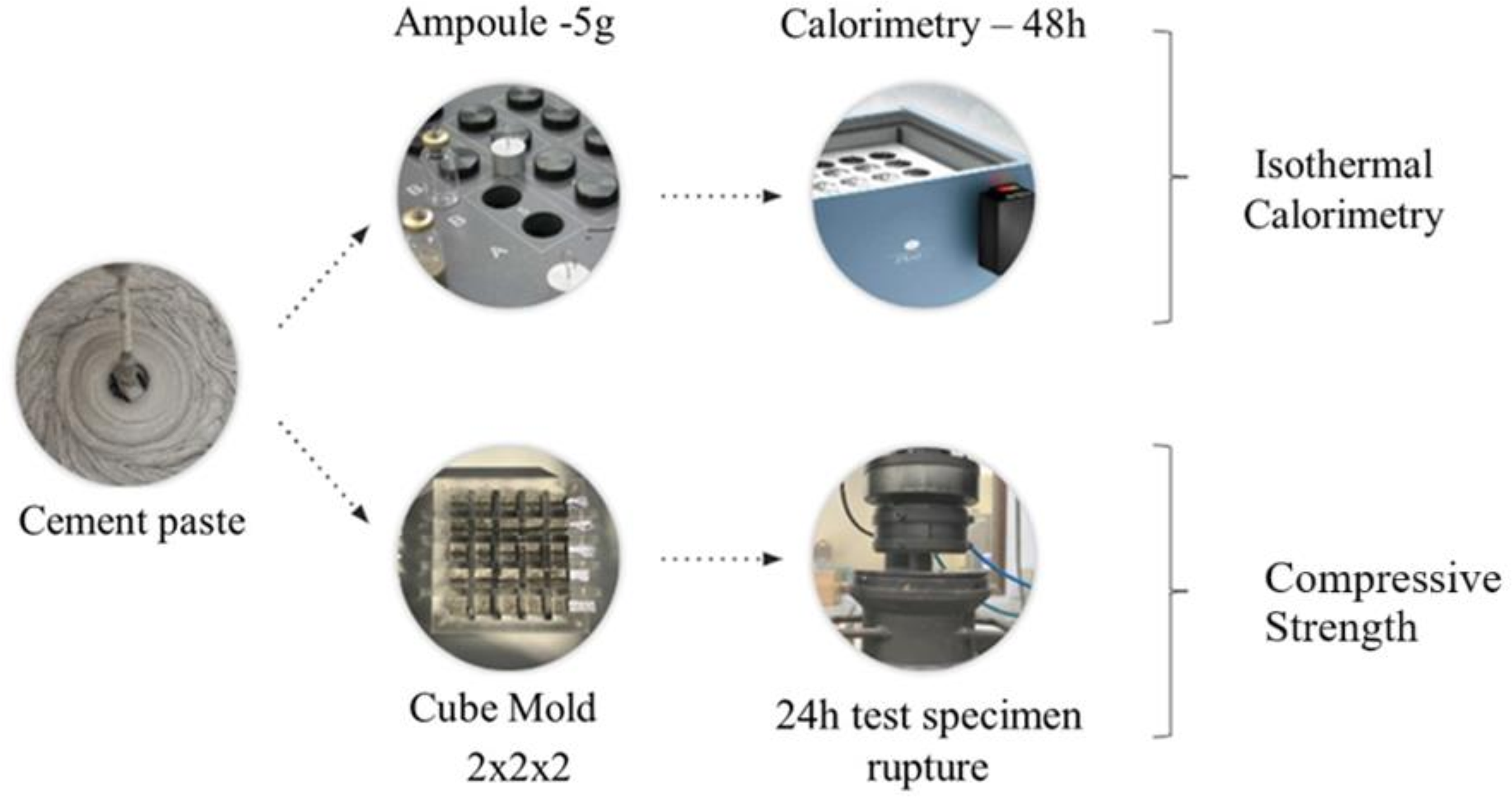
Cement pastes were produced with a water-to-cement ratio of 0.5 to evaluate the influence of the Potential Alternative Accelerators (PA) on the hydration of Portland cement made with PG. The PAs were dissolved in mixing water and stirred with a magnetic stirrer for 5 minutes before being added to the cement. The hydration of the cement pastes was assessed in fresh and hardened states, following the experimental program described in Figure 1. The pastes were mixed for 2 minutes in an automatic mixer at 10,000 rpm. Additionally, the cement pastes were identified (ID) in two parts: type of accelerator and percentage of the accelerator (by cement mass) – for instance, NaOH-0.3.
Isothermal calorimetry
Isothermal calorimetry tests were carried out for 48 hours on fresh pastes (1 unit per mix) in a TA Instruments 8-channel TAM AIR isothermal calorimeter at a constant temperature of 25 ± 0.05 ºC following C1679 (ASTM, 2017). Reference ampoules used water with a mass adjusted to have a calorific value compatible with the cement paste samples. Additionally, the cement and mixing water were stored previously for 24 hours in the calorimeter´s room to equalize their temperature to 25 ºC. The results of heat flow and heat release (mW/g) were normalized by the mass of the paste added to the ampoule. Parameters of the heat flow curves were also defined for comparative purposes, including (1) cumulative heat at 24 hours; (2) time to the start of the acceleration period in the heat flow curves; (3) time to reach the peak of the heat flow curve, as shown in Figure 2.
Compressive strength
Compressive strength tests were performed (5 units per mix and age) on hardened cement pastes using an EMIC DL20000 press with a 200 kN load cell, precision of 1N, and a loading rate of 0.25 mm/min. These equipment settings and the number of specimens were based on previous tests. The cubic-shaped specimens (CS) tested of 2x2x2 cm were molded in acrylic forms, as shown in Figure 1. After molding, the acrylic forms with the samples were wrapped in plastic film and cured in a humidity chamber of 95% until the testing age. Finally, the specimens had their testing surfaces polished to avoid stress concentration points. The choice of smaller-sized specimens was made due to the high material consumption in conventional compressive strength tests. The specimens were tested 24h after molding.
Additionally, it was desired to evaluate the behavior of the cement paste alone without the influence of aggregates. A similar methodology was adopted in other studies (Costa, 2013; Longhi, 2015). Statistical two-way analysis of variance (ANOVA) was performed on the compressive strength data.
Results and discussions
Isothermal calorimetry
The heat flow curve of a typical cement is composed of five main parts, as shown in Figure 2 (Mostafa; Brown, 2005):
-
I - the initial reaction;
-
II - the induction period;
-
III – the acceleratory period;
-
IV – The decelerator period; and
-
V – the period of slow continued reaction.
These parts are visualized in the heat flow curves of Figure 3, and the sulfate depletion point (E) in some of the curves is noted, where there is the resumption of the aluminate reaction. The initial reaction is related to the dissolution of ions when cement comes in contact with water, showing a great exothermic peak of heat release. The induction period is a low heat-releasing period. The acceleratory period is mainly related to C3S hydrated product formation, C-S-H, and CH. The deceleratory period is a decrease in the heat released and depletion of added calcium sulfate from the system, known as sulfate depletion (E).
Heat flow for 48 hours of the cement pastes produced with PA: (a) CaCl2; (b) NaCl; (c) Na2SiO3; (d) NaOH
The results of the curves in Figure 3 suggest that all PA tested accelerated the hydration reactions in the initial hours of the cement tested. However, each PA exhibited different levels of efficiency. Samples with CaCl2 and NaCl exhibited higher heat flow curves than REF, indicating higher heat flows during the acceleration period of the main peak. This difference was approximately 0.25 mW/g for CaCl2 and 0.5 mW/g for NaCl compared to REF. Although both materials are chloride-based, Zhu et al. (2023) emphasized that the influence of chloride salts is highly dependent on the type of cation with which it is associated. However, for the compounds evaluated in this study, the Ca2+ cation would theoretically have a more significant impact on accelerating reactions than Na+, according to molarity conditions (Dorn; Blask; Stephan, 2022). This behavior is not as substantial in the heat flow curves in Figure 3(a) and 3(b). Similarly, the reaction of cement pastes with NaOH was accelerated, and the intensity of the main peak in the curves was higher than the REF curve, Figure 3(d).
Vehmas, Kronlöf, and Cwirzen (2018), when evaluating CaCl2 as an accelerator in cement, also found that it accelerated hydration over the 24-hour measurement period. Additionally, Mota, Matschei, and Scrivener (2018) observed the acceleration of hydration and gain in strength in the early hours for white cement tested with NaOH, although NaOH later reduced the strength. Anchez-Herrero, Fernandez-Jimenez, and Palomo (2015), when hydrating C3S in alkaline media with 8-M NaOH, did not find significant effects on strength gain. On the other hand, the hydration of C2S in this alkaline media significantly accelerated its hydration, impacting setting and hardening times.
Conversely, samples containing Na2SiO3, while displaying heat flow curves preceding that of the reference sample, demonstrate a reduced intensity in the primary hydration peak compared to the REF curve. The intensity of the principal peak in the curves of samples with Na2SiO3 decreases with increasing Na2SiO3.
It is emphasized that the use of alkali-containing accelerators can cause long-term strength loss, compromise dimensional stability, and increase the risk of alkali-aggregate reaction (if aggregates are reactive), affecting durability when used in high dosages (above 10-20%) (Wang et al., 2021, 2022). In the case of sodium silicate-based accelerators, dosages above 10% may even compromise adhesion to the substrate in shotcrete applications (Wang et al., 2021). Nevertheless, employing reduced contents, integrating supplementary cementitious materials, and incorporating chemical water-reducing admixtures stand as viable alternatives to mitigate these challenges (Wang et al., 2021, 2022).
Furthermore, it is observed in the heat flow curves in Figure 3 that the sulfate balance of the cement is affected using this PA. Many factors can affect the sulfate demand in cement, and chemical admixtures, such as accelerators and plasticizers, are highlighted as one of them (Andrade Neto; De La Torre; Kirchheim, 2021b). If the cement is not adequately sulfated, the admixture highlights this characteristic. An under-sulfated cement happens when the calcium sulfate source does not provide enough sulfates to delay the aluminate hydration or the presence of the admixture suppresses the availability rate of these sulfates (Cheung et al., 2011). In the case of REF, the resumption of aluminate reactions (E) is evident after the main peak associated with silicate reactions, indicating that this cement is adequately sulfated. Cement is considered adequately sulfated when sulfate exhaustion occurs after the main peak associated with silicate reactions (Andrade Neto; De La Torre; Kirchheim, 2021a). However, the sulfate exhaustion point (E) was not visualized in the curves in Figure 3(a), 3(b), and 3(c), suggesting that sulfate exhaustion occurred before the main peak associated with silicate reactions, a behavior not observed in REF.
In the dosages with NaOH, point (E) was noticed and anticipated with the increase in the potential accelerator (PA) contents compared to REF. The result suggested that the sulfate balance of the evaluated cement was affected by the presence of PA. Other factors, such as temperature, the presence of supplementary cementitious materials (SCM), and other materials, also interfere with the accelerator’s effect, highlighting the complexity of these systems (Cheung et al., 2011).
Regarding the curves of cumulative heat up to 48 hours, it was observed in Figure 4 that the highest values are reached by pastes with NaOH, followed by those with NaCl. Furthermore, the cumulative heat is always higher than the REF value during the first 48 hours for these samples. In contrast, samples with CaCl2 and Na2SiO3 showed results that varied (lower or higher) compared to REF over the 48 hours. These samples exhibited higher cumulative heat than REF before 24 hours, although they demonstrated lower cumulative heat than REF, at some dosages, after this period. Therefore, there was a trend change in cumulative heat for CaCl2 and Na2SiO3 samples over the analyzed 48 hours. The trend of the PA changed approximately after 24 hours for these samples, while it remained similar for NaOH and NaCl.
Cumulative heat for 48 hours of the cement pastes produced with PA: (a) CaCl2; (b) NaCl; (c) Na2SiO3; (d) NaOH
In Figures 5, 6, and 7, selected parameters of the heat curves are displayed:
-
cumulative heat at 24 hours;
-
time to initiate the acceleration period in the heat flow curves; and
-
time to reach the peak of the heat flow curve.
The cumulative heat at 24 hours (1) was selected to compare with the compressive strength data of the pastes at 24 hours since the cumulative heat indicates the strength gain. On the other hand, the time to initiate the acceleration period (2) and time to reach the peak (3) of the heat flow curves were chosen as indicators of the reaction rates, which are a factor that impact the setting time.
The highest values of (1) were achieved with NaOH followed by NaCl, while Na2SiO3 and CaCl2 showed inferior performance. This trend was initially observed in the cumulative heat curves in Figure 4; now, in Figure 5, it becomes more evident. The Na2SiO3-1.5% and Na2SiO3-2.0% samples (higher dosages) presented values of (1) that are 2.4% and 2% lower compared to REF. However, all NaOH, NaCl, and CaCl2 dosages showed (1) higher than REF. The highest (1) was achieved with NaOH-1.00, 29.49% higher than REF. Overall, the (1) of the NaOH samples was approximately 24% (considering the average value) higher than REF. Meanwhile, the (1) of the NaCl samples remained around 141-143 J/g with an average value approximately 12.5% higher than REF. The CaCl2 samples exhibited similar behavior with values ranging between 129-132 J/g and an average value approximately 4% higher than REF. Also, Figure 5 shows that the lower cumulative heat observed in the Na2SiO3-1.5% and Na2SiO3-2.0% samples at 24 hours were not observed before 24 hours. All Na2SiO3 samples showed cumulative heat superior to REF before 24 hours. However, this behavior changed for Na2SiO3 around 20 hours and 24 hours for the samples with CaCl2. Regardless of the dosage, NaOH and NaCl enhanced the cumulative heat until 48 hours. Vehmas, Kronlöf, and Cwirzen (2018) suggested, through thermodynamic modeling, that CaCl2 provides supersaturation relative to calcium silicate hydrate (C-S-H), and this supersaturation is responsible for the chloride-induced acceleration.
Regarding (2) and (3) - Figures 6 and 7 -, in general, the times were reduced with the increments of PA, except for the samples with NaOH and CaCl2 in (2). The dosages that managed to reduce (2) below the times of REF were: REF (2.41h) > CaCl2 - 0.75 (2.31h) > Na2SiO3 - 1.0 (2.25h) > Na2SiO3 - 0.5 (2.22h)> Na2SiO3 - 1.5 (2.12h) > Na2SiO3 - 2.0 (1.96h). On the other hand, for (3), except the NaOH-0.25 (11.98h) and Na2SiO3-0.5 (11.52h) samples, all samples showed values below REF (11.40h).
The pastes tested with Cl- anion-based PA (CaCl2 and NaCl) demonstrated better performance by reducing the time to reach the peak of the acceleration curve (3), suggesting fundamental mechanisms to accelerate reactions in silicate phases. In the samples based on the Cl- anion in (2), only CaCl2-0.75 (2.31h) was reduced in the acceleration period compared to REF (2.41h), and this difference was 4%. Thus, CaCl2 and NaCl were more effective in anticipating the acceleration and induction periods. NaCl samples vary between 9.63-10.4h, with values on average 12% lower than REF. Similarly, higher dosages of these materials resulted in reduced timeframes. In contrast, Na2SiO3 was 19% more effective in reducing (2) than REF. Finally, NaOH was ineffective in anticipating the induction period (2), showing a delay of 20%. NaOH-0.25 did not reduce the acceleration period (3), showing curves 5% higher than REF, while the other dosages presented lower results than REF. This result suggests that the tested PA was more effective in reducing the time to reach the peak (3) in the acceleration period of the curves (approximately 3-12h) than in reducing the time to start the acceleration period (2) in the induction period (approximately 0.5-3h).
Compressive strength
Figure 8, one can note that the Na2SiO3, regardless of the dosage, presented a lower efficiency than the other PA. Guo et al. (2018) studied the effect of sodium silicate in cement pastes containing lead and zinc smelting slag at 0.4%. The Na2SiO3 gel accelerated the hydration process of the binder, increased cement hydration products, refined the cement pore structure, and increased the compressive strength of the cement. However, at 1 day, they observed a reduction in initial strength similar to what occurred in the samples of this study. It is worth noting that the materials composing the cement, as well as the exposed environment, can influence the accelerator’s effect (Saedi; Jamshidi-Zanjani; Darban, 2021; Wang; Duong; Zhang, 2023).
Compressive strength of cement pastes with 24h composed of clinker, phosphogypsum, and accelerators
In contrast, higher strengths were obtained with NaOH; all dosages exhibited higher values than REF. This trend corroborates the results observed for cumulative heat. Mota, Matschei, and Scrivener (2018) also verified the increase in initial strengths for cement pastes with NaOH.
The CaCl2 content displayed consistent compressive strength, with a maximum difference of 2 MPa between the highest and lowest values obtained. Additionally, the different dosages used performed closely to the REF sample. Regarding NaCl, the 0.3% dosage exhibited the least satisfactory performance, even falling below the REF. However, increasing the dosage to 0.45% showed a trend toward excellence standards observed for NaOH dosages. As NaCl dosages increased (0.6% and 0.75%), achieved strengths decreased, yet they remained above the reference value. Wang, Duong, and Zhang (2023) discussed that chloride-based accelerators increase the compressive strength of cement. The authors link the strength gain to the acceleration mechanism of these materials, where chloride essentially speeds up the hydration of C3S, increasing the dissolution of C3S in the pore solution, and more permeable C-S-H can be formed at very early ages. Yuan et al. (2020) also highlighted that CaCl2, at concentrations of 1% and 2%, notably enhanced strength in cement pastes by accelerating reactions right after the induction period, thereby shortening this phase. This phenomenon is also depicted in Figure 3.
These results supported the trend from the analysis of variance (ANOVA) of the compressive strength data, which suggested that the accelerator type, dosage, and interaction influenced the compressive strength response (24h), as shown in Table 4. It is worth noting, however, that a mean data comparison was not performed.
Conclusions
This study assessed sodium chloride (NaCl), calcium chloride (CaCl2), sodium hydroxide (NaOH), and sodium silicate (Na2SiO3) as potential alternative accelerators (PA) to enhance early strength gain and hydration rates in cement using 100% phosphogypsum (PG) as the calcium sulfate source, replacing non-renewable natural gypsum. Exploring the feasibility of utilizing industrial wastes to replace non-renewable natural resources from other industries is crucial for sustainable solutions, particularly cement production. Overcoming challenges such as setting delays and reduced strength is essential to fully leveraging the potential of phosphogypsum. Research into these challenges uncovers various approaches, with accelerators emerging as a promising solution.
The results highlighted variations in the performance of the tested accelerators regarding early strength gain and hydration rates. Pastes incorporating NaOH exhibited higher strength values and cumulative heat at 24 hours than other accelerators, suggesting its potential as a strength enhancer. Despite accelerating the sulfate depletion point (E) similar to the reference (REF), NaOH does not disrupt the sulfate balance of the cement. On the other hand, Na2SiO3 accelerated setting during the induction period (2), preceding reactions compared to REF but affecting 24-hour strength with lower cumulative heat values (24h) than other samples. Adjusting dosages may mitigate these effects on strength.
CaCl2 demonstrated strengths and reaction rates similar to REF. Conversely, NaCl showed higher strengths and cumulative heat (24h) than REF, indicating its effectiveness as an accelerator. However, it is crucial to consider durability studies due to the risk of reinforcement corrosion despite chloride ion levels being within standard limits. These findings underscore the importance of carefully evaluating accelerators for optimizing cement mixtures containing phosphogypsum, balancing performance gains with long-term durability considerations.
References
- AKFAS, f. et al. Exploring the potential reuse of phosphogypsum: a waste or a resource? Science of the Total Environment, v. 908, p. 168196, 2024.
- AMERICAN SOCIETY FOR TESTING MATERIALS. C1679: measuring hydration kinetics of hydraulic cementitious mixtures using isothermal calorimetry. West Conshohocken, 2017.
- AMERICAN SOCIETY FOR TESTING MATERIALS. C563-20: standard guide for approximation of optimum so3 in hydraulic cement. West Conshohocken, 2020.
- ANCHEZ-HERRERO, S.; FERNANDEZ-JIMENEZ, A.; PALOMO, A. Alkaline hydration of c2s and c3s. Journal of the American Ceramic Society, v. 99, n. 2, p. 604-611, 2015.
- ANDRADE NETO, J. da S.; DE LA TORRE, A. G.; KIRCHHEIM, A. P. Effects of sulfates on the hydration of portland cement: a review. Construction and Building Materials, v. 279, p. 122428, 2021a.
- ANDRADE NETO, J. S. et al. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Construction and Building Materials, v. 299, p. 123935, 2021b.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 11579: cimento portland: determinação do índice de finura por meio da peneira 75 μm (nº 200). Rio de Janeiro, 2012.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 12655: concreto de cimento portland: preparo, controle, recebimento e aceitação: procedimento. Rio de Janeiro, 2015a.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16372: cimento portland e outros materiais em pó: determinação da finura pelo método de permeabilidade ao ar (método blaine). Rio de Janeiro, 2015b.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16606: cimento portland: determinação da pasta de consistência normal. Rio de Janeiro, 2018c.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16607: cimento portland: determinação dos tempos de pega. Rio de Janeiro, 2018b.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16697: cimento portland: requisitos. Rio de Janeiro, 2018a.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 17086-6: cimento portland: análise química: parte 6: determinação da perda ao fogo. Rio de Janeiro, 2023.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7215: cimento portland: determinação da resistência à compressão de corpos de provas cilíndricos. Rio de Janeiro, 2019.
- BÉNARD, P. et al. Hydration process and rheological properties of cement pastes modified by orthophosphate addition. Journal of the European Ceramic Society, v. 25, n. 11, p. 1877–1883, 2005.
- BÉNARD, P. et al. Influence of orthophosphate ions on the dissolution of tricalcium silicate. Cement and Concrete Research, v. 38, n. 10, p. 1137–1141, 2008.
- CÁNOVAS, C. R. et al. Exploration of fertilizer industry wastes as potential source of critical raw materials. Journal of Cleaner Production, v. 143, p. 497–505, 2017.
- CÁNOVAS, C. R. et al. Valorization of wastes from the fertilizer industry: current status and future trends. Journal of Cleaner Production, v. 174, p. 678–690, 2018.
- CANUT, M. M. C. et al. Microstructural analyses of phosphogypsum generated by brazilian fertilizer industries. Materials Characterization, v. 59, n. 4, p. 365–373, 2008.
- CAO, J. et al. Promoting coordinative development of phosphogypsum resources reuse through a novel integrated approach: a case study from China. Journal of Cleaner Production, v. 374, p. 134078, 2022a.
- CAO, W. et al. Effect of soluble fluorine on the hydration, strength and microstructure of hemihydrate phosphogypsum. Journal of Environmental Chemical Engineering, v. 12, n. 1, p. 111890, 2024.
- CAO, W. et al. Preparation of anhydrite from phosphogypsum: influence of phosphorus and fluorine impurities on the performances. Construction and Building Materials, v. 318, p. 126021, 2022b.
- CHEUNG, J. et al. Impact of admixtures on the hydration kinetics of portland cement. Cement and Concrete Research, v. 41, n. 12, p. 1289–1309, 2011.
- COSTA, A. R. D. et al. Hydration of sustainable ternary cements containing phosphogypsum. Sustainable Materials and Technologies, v. 28, p. e00280, 2021.
- COSTA, E. B. da. Aproveitamento do resíduo de anodização do alumínio na produção de cimento sulfoaluminato de cálcio belítico Porto Alegre, 2013. Dissertação (Mestrado em Engenharia Civil) – Universidade Federal do Rio Grande do Sul, Porto Alegre, 2013.
- COSTA, R. P. et al. Effect of soluble phosphate, fluoride, and ph in brazilian phosphogypsum used as setting retarder on Portland cement hydration. Case Studies in Construction Materials, v. 17, p. e01413, 2022.
- CUI, Y. et al. A systematic review of phosphogypsum recycling industry based on the survey data in china-applications, drivers, obstacles, and solutions. Environmental Impact Assessment Review, v. 105, p. 107405, 2023.
- DERAKHSHANI, A.; GHADI, A.; EBRAHIM VAHDAT, S. Study of the effect of calcium nitrate, calcium formate, triethanolamine, and triisopropanolamine on compressive strength of portland-pozzolana cement. Cases Studies in Construction Materials, v. 18, p. e01799, 2023.
- DORN, T.; BLASK, O.; STEPHAN, D. Acceleration of cement hydration: a review of the working mechanisms, effects on setting time, and compressive strength development of accelerating admixtures. Construction and Building Materials, v. 323, p. 126554, 2022.
- ENNACIRI, Y.; BETTACH, M. The chemical behavior of the different impurities present in phosphogypsum: a review. Phosphorus, Sulfur and Silicon and the Related Elements, v. 99, n. 2, p. 129-148, 2024.
- GUO, L. et al. Sodium silicate gel effect on cemented tailing backfill that contains lead-zinc smelting slag at early ages. Advances in Materials Science and Engineering, v. 8, 2018.
- HANEKLAUS, N. et al. Closing the upcoming eu gypsum gap with phosphogypsum. Resources, Conservation & Recycling, v. 182, 2022.
- HERMANN, L.; KRAUS, F.; HERMANN, R. Phosphorus processing-potentials for higher efficiency. Sustainability, v. 10, n. 5, 2018.
- HOLANDA, F. do C.; SCHMIDT, H.; QUARCIONI, V. A. Influence of phosphorus from phosphogypsum on the initial hydration of portland cement in the presence of superplasticizers. Cement and Concrete Composites, v. 83, p. 384–393, 2017.
- HOSEIN, S.; MOUSAVINEJAD, G.; SAMMAK, M. An assessment of the effect of na2sio3 /naoh ratio, naoh solution concentration, and aging on the fracture properties of ultra-high-performance geopolymer concrete: the application of the work of fracture and size effect methods. Structures, v. 39, p. 434–443, 2022.
- JIA, R.; WANG, Q.; LUO, T. Reuse of phosphogypsum as hemihydrate gypsum: the negative effect and content control of h3po4. Resources, Conservation and Recycling, v. 174, 2021.
- KIRCHHEIM, A. P. et al. Alkalis incorporated into tricalcium aluminate: effects in hydration. Ambiente Construído, Porto Alegre, v. 10, n. 1, p. 177–189, jan./mar. 2010.
- LI, W. et al. Effects of seawater, nacl, and na2so4 solution mixing on hydration process of cement paste. Journal of Materials in Civil Engineering, v. 33, n. 5, 2021.
- LONGHI, M. A. Álcali-ativação de lodo de caulim calcinado e cinza pesada com ativadores convencionais e silicato de sódio alternativo Porto Alegre, 2015. Dissertação (Mestrado em Engenharia Civil) – Universidade Federal do Rio Grande do Sul, Porto Alegre, 2015.
- LU, Z. et al. Towards a further understanding of cement hydration in the presence of triethanolamine. Cement and Concrete Research, v. 132, 2020.
- MAAZOUN, H.; BOUASSIDA, M. Tunisian phosphogypsum challenges. Geotechnical Engineering Journal of the Seags & Agssea, v. 51, n. 4, 2020.
- MEHTA, P. K.; MONTEIRO, P. J. M. Concrete: microstructure, properties and materials 3rd ed. New York: Mcgraw-hill Education, 2014.
- MOSTAFA, N. Y.; BROWN, P. W. Heat of hydration of high reactive pozzolans in blended cements: isothermal conduction calorimetry. Thermochimica Acta, v. 435, n. 2, p. 162–167, 2005.
- MOTA, B.; MATSCHEI, T.; SCRIVENER, K. Impact of naoh and na2so4 on the kinetics and microstructural development of white cement hydration. Cement and Concrete Research, v. 108, p. 172–185, 2018.
- QAMOUCHE, K. et al. Radiological characterization of phosphate rocks, phosphogypsum, phosphoric acid and phosphate fertilizers in Morocco: an assessment of the radiological hazard impact on the environment. Materials Today: Proceedings v. 27, p. 3234-3242, 2020.
- QI, H. et al. Influence of fluoride ion on the performance of pce in hemihydrate gypsum pastes. Journal of Building Engineering, v. 46, p. 103582, 2022.
- QIN, X. et al. Resource utilization and development of phosphogypsum-based materials in civil engineering. Journal of Cleaner Production, v. 387, p. 135858, 2023.
- PRUDHCIO JUNIOR, L. R. Accelerating admixtures for shotcrete. Cement and Concrete Composites, v. 20, p. 213–219, 1998.
- RADHOUAN, E. Z. et al. Characterization of the role of phosphogypsum foam in the transport of metals and radionuclides in the southern mediterranean sea. Journal of Hazardous Materials, v. 363, p. 258–267, 2019.
- RAMACHANDRAN, V. S. Accelerators. In: CONCRETE admixtures handbook properties, science, and technology. 2nd ed. New Jersey: William Andrew, 1996.
- RAMTEKE, L. P. et al. Study of fluoride content in some commercial phosphate fertilizers. Journal of Fluorine Chemistry, v. 210, p. 149–155, 2018.
- RODRIGUES LAGO, F. et al. Evaluation of influence of salt in the cement hydration to oil wells. Materials Research, v. 20, p. 743–747, 2017.
- SAEDI, A.; JAMSHIDI-ZANJANI, A.; DARBAN, A. K. A review of additives used in the cemented paste tailings: environmental aspects and application. Journal of Environmental Management, v. 289, p. 112501, 2021.
- TABIKN, A. A.; MILLER, F. M. The nature of phosphogypsum impurities and their influence on cement hydration. Cement and Concrete Research, v. 1, p. 663–678, 1971.
- TAYIBI, H. et al. Environmental impact and management of phosphogypsum. Journal of Environmental Management, v. 90, p. 2377–2386, 2009.
- TAYLOR, H. F. W. Cement chemistry London: Academic Press, 1990.
- VEHMAS, T.; KRONLÖF, A.; CWIRZEN, A. Calcium chloride acceleration in ordinary Portland cement. Magazine of Concrete Research, v. 70, n. 16, p. 856–863, 2018.
- VIEIRA ALVES, C. Cloreto de sódio como acelerador em cimentos com fosfogessos: análise de resistência à compressão e potencial de corrosão. Porto Alegre, 2021. Trabalho de Conclusão de Curso (Bacharelado em Engenharia Civil) – Universidade Federal do Rio Grande do Sul, Porto Alegre, 2021.
- WANG, C.; CHEN, X.; WANG, R. Do chlorides qualify as accelerators for the cement of deepwater oil wells at low temperature? Construction and Building Materials, v. 133, p. 482–494, 2016.
- WANG, W. C.; DUONG, H. T. H.; ZHANG, C. H. Influence of accelerating admixtures on high early strength cement performance using heat curing method. Case Studies in Construction Materials, v. 18, 2023.
- WANG, Y. et al. Accelerators for normal concrete: a critical review on hydration, microstructure and properties of cement-based materials. Cement and Concrete Composites, v. 134, p. 104762, 2022.
- WANG, Y. et al. Accelerators for shotcrete-chemical composition and their effects on hydration, microstructure and properties of cement-based materials. Construction and Building Materials, v. 281, p. 122557, 2021.
- XIE, H. et al. Effect of complexation of alkanolamine in accelerators on the initial stage of cement hydration. Construction and Building Materials, v. 393, p. 1321052023, 2023.
- XU, Z. et al. Research on cement hydration and hardening with different alkanolamines. Construction and Building Materials, v. 141, p. 296-30615, 2017.
- YANG, J. et al. Effect of na2co3 on the tensile creep of slag-fly ash systems activated with na2sio3. Cement and Concrete Composites, v. 140, p. 105110, 2023.
- YUAN, Q. et al. Structural build-up, hydration and strength development of cement-based materials with accelerators. Construction and Building Materials, v. 259, 2020.
- ZHANG, J. et al. Effect of soluble p2o5 form on the hydration and hardening of hemihydrate phosphogypsum. Advances in Materials Science and Engineering, v. 2022, 2022.
- ZHU, G. et al. Effects of chloride salts on strength, hydration, and microstructure of cemented tailings backfill with one-part alkali-activated slag. Construction AND Building Materials, v. 374, p. 130965, 2023.
Edited by
-
Editor:
Marcelo Henrique Farias de Medeiros
Publication Dates
-
Publication in this collection
10 Mar 2025 -
Date of issue
Jan-Dec 2025
History
-
Received
13 Mar 2024 -
Accepted
25 June 2024

 Influence of alternative accelerators on the hydration and strength of Portland cement with phosphogypsum
Influence of alternative accelerators on the hydration and strength of Portland cement with phosphogypsum