Abstract
This research examines the effects of different calcium sulfate combinations—natural gypsum (NG), untreated phosphogypsum (PG), and lime-treated phosphogypsum (NPG)—on the hydration and rheology of Portland cement with a lignosulfonate admixture. Three novel calcium sulfate compositions (SU I, II, III) were evaluated as setting regulators in Portland cement types CEM I, II, and III, focusing on setting times, compressive strength, heat release, hydration products, and rheological behavior. The compositions are: SU I (30% PG, 70% NG), SU II (50% NPG, 50% NG), and SU III (100% NPG). Results showed that CEM III had lower early strength and longer setting times than CEM I and II, effects amplified by the lignosulfonate admixture. Rheological differences among cements were attributed to PG’s delayed hydration, reducing yield stress and viscosity. The study highlights how cement composition, alternative calcium sulfates, and admixtures interact to affect fresh and hardened cement properties.
Keywords
Phosphogypsum; Gypsum; Lignosulfonate; Hydration; Rheology
Resumo
Esta pesquisa examina os efeitos de diferentes combinações de sulfato de cálcio – gipsita natural (NG), fosfogesso não tratado (PG) e fosfogesso tratado com cal (NPG) – na hidratação e reologia do cimento Portland com um aditivo lignossulfonato. Três novas composições de sulfato de cálcio (SU I, II e III) foram avaliadas como reguladores de pega nos cimentos Portland dos tipos CEM I, II e III, com foco nos tempos de início e fim de pega, resistência à compressão, liberação de calor, produtos de hidratação e comportamento reológico. As composições são: SU I (30% PG, 70% NG), SU II (50% NPG, 50% NG) e SU III (100% NPG). Os resultados mostraram que o CEM III apresentou menor resistência inicial e tempos de pega mais longos em comparação aos cimentos CEM I e II, efeitos amplificados pelo aditivo lignossulfonato. Diferenças reológicas entre os cimentos foram atribuídas à hidratação retardada pelo PG, reduzindo a tensão de escoamento e a viscosidade. O estudo destaca como a composição do cimento, sulfatos de cálcio alternativos e aditivos interagem para influenciar as propriedades do cimento no estado fresco e endurecido.
Palavras-chave
Fosfogesso; Gipsita; Lignossulfonato; Hidratação; Reologia
Introduction
The growing population makes fertilizers crucial for meeting the increasing demand for food and maintaining agricultural productivity. However, as with other processes, fertilizers produce large amounts of waste that can harm living organisms and the environment (Silva et al., 2022). The acid phosphoric production used to produce phosphorus fertilizers is included in this scenario. On the other hand, in the context of the modern circular economy, there is a pressing need for proper waste management and efficient integration of waste into the global economy.
Approximately 85% of phosphate rock is converted into phosphoric acid using the wet or thermal process (Zan et al., 2006). The wet method is known for its superior energy efficiency compared to the thermal method, making it the more prevalent option. Approximately 71% of the total phosphate rock production with sulfuric acid is transformed into phosphoric acid through a wet process. However, a solid waste called phosphogypsum (PG) is generated in this process. The production of one ton of phosphoric acid generates about 4 to 5 tons of PG by-products. PG production rates will follow fertilizer production rates, projecting a sevenfold increase by 2050 compared to 1961, reaching 438 million tons per year (Akfas et al., 2024; Tayibi et al., 2009).
The type of phosphate rock, the phosphoric acid production process, industrial efficiency, storage, and the chemical quality of the applied reagents influence PG. These factors directly affect PG’s impurities, mineralogical, morphology, and particle size (Guerrero‐Márquez et al., 2017). Essentially, PG is composed of gypsum (CaSO4∙2H2O). Still, it can also contain minor impurities such as phosphate, fluorine, heavy metals, residual acid, natural radionuclides, rare earth elements (REEs), and other trace elements (Abril; García-Tenorio; Manjón, 2009; Rashad, 2017).
Due to its main composition being calcium sulfate (SU), PG is a promising material for construction materials, especially in Portland cement, as a substitute for natural gypsum (NG) (Chen et al., 2022; Costa; Gonçalves, 2022; Qin et al., 2023). Also, PG is an environmental friendly viable alternative in Brazil, considering that 93% of natural gypsum sources are in the Northeast region, making transportation to the southern region costly (Canut et al., 2008; Costa et al., 2022; Rashad, 2017). According to Costa et al. (2022), Brazilian PG is categorized as an acidic source of calcium sulfate. Additionally, alkaline PG results from practices such as rinsing PG with tap water or adding lime, which makes the pH range between 8 and 12.
Specifically in cement production, PG and NG, both materials, control the hydration reactions of tricalcium aluminate (C3A), one of the most reactive phases of Portland clinker, and prevent rapid setting reactions in cement. However, due to the presence of fluorine (F-), phosphates (P2O5), and acid pH, PG results in undesired prolongation of setting times and reduction in the initial mechanical strength of cementitious mixtures (Costa et al., 2022; Tabikn; Miller, 1971).
Xu et al. (2023) suggest that phosphorus is the primary harmful impurity for cement hydration presented in PG. According to the authors, phosphorus is present in soluble, eutectic, and insoluble forms, where the soluble form, present in the form of H3PO4, H2PO4-, and HPO2−, mainly impacts the performance of PG with binders or as a binder (Jia; Wang; Luo, 2021; Zhang et al., 2022). When evaluating the soluble forms of P2O5, - H3PO4; H2PO42−; and HPO42−− Zhang et al. (2022) assert that HPO42− significantly delays the initial hydration reaction, prolongs setting time, and reduces mechanical performance. Also, Holanda, Schmidt, and Quarcioni (2017) point out that concentrations of P2O5 between 0.83% and 1.64% significantly impact the initial hydration. Furthermore, other studies consider the delay in hydration in cement with PG happens because soluble fluorine and phosphorus react with calcium ion precipitating CaF2 and Ca3(PO4)2, reducing the hydration reaction of cement (Bénard et al., 2005, 2008; Singh, 2002; Tabikn; Miller, 1971).
Moreover, to mitigate the retardation effect in cement hydration and lower strength gain at early ages caused by the impurities mentioned above from PG, physical, chemical, thermal, and combinations of treatments are suggested in the literature (Andrade Neto et al., 2021; Cai et al., 2021; Cao et al., 2021; Li; Zhang, 2021; Lv; Xiang, 2023; Palla et al., 2022; Rosales et al., 2019). Among chemical treatments, the addition of hydrated lime to phosphogypsum or washing with dissolved lime have been commonly employed (Andrade Neto et al., 2021; Chen et al., 2018; Liu; Wang; Yu, 2019). A study treating PG with lime to neutralize impurities shows that OH−, instead of Ca2+ from calcium hydroxide (CH), mainly affects the initial reaction. Dissolved OH− causes the precipitation of Al3+, Fe3+, PO43-, and F- with pH change, resulting in a layer around PG. Also, using CH to treat PG acts as crystallization points and provides adequate Ca2+ for the reaction (Cui et al., 2022).
Additionally, the feasibility of the chosen treatment on an industrial scale, associated costs, and the secondary wastes generated by the treatment requires careful consideration. Treating PG impurities must not be prohibitively expensive, rendering PG nonviable as a setting regulator in the cement matrix. Therefore, the solid hydrated lime neutralization of PG presents a promising alternative, as the chemical is cost-effective compared to other options, generates no waste, and requires minimal modification of existing cement industry infrastructure. To thoroughly assess the advantages and drawbacks of utilizing PG, this study proposes three novel compositions of calcium sulfate (SU) sources comprising untreated phosphogypsum (PG), natural gypsum (NG), and phosphogypsum neutralized with 5% solid hydrated lime (NPG) in varying proportions. The novel compositions include SU I (30% PG and 70% NG), SU II (50% NPG and 50% NG), and SU III (100% NPG).
Furthermore, adding chemical admixture complicates the cement matrix system with PG (Andrade Neto et al., 2021; Holanda; Schmidt; Quarcioni, 2017; Qi et al., 2022). A few studies have assessed the behavior of polycarboxylate and lignosulfonate superplasticizers in Portland cement with PG (Andrade Neto et al., 2021; Holanda; Schmidt; Quarcioni, 2017). However, there is still a lack of information on this theme. Most of the studies are related to using chemical admixtures with anhydrite and hemihydrate gypsum pastes made with calcinated phosphogypsum (Qi et al., 2022). Also, there is still divergence regarding the efficiency of the hydrated lime treatments in the presence of chemical admixture in Portland cement pastes and its effects on hydration.
Regarding rheology, chemical admixture is used to improve the workability of the matrix and reduce the water/binder ratio, making their compatibility with the materials used essential. Since the impurities present in phosphogypsum can interact with the polymer, compromising its dispersing power (Qi et al., 2022). Thus, understanding the behavior of PG in the presence of these admixtures is essential to make its use as a setting regulator viable.
In this study, a Lignosulfonate (LS) admixture is assessed. LS is a material from the pulp and paper industry that is used as a water reducer in concrete. Also, other cations, such as Ca+2, Na+2, and Mg+2 can form complex structures with LS (Arel; Aydin, 2017). Lignosulfonate is one the most representative water reducers for concrete on the Brazilian market. The water reducer application for lignosulfonate is possible because lignosulfonate contains hydrophilic groups (sulphonic, phenylic hydroxyl, and alcoholic hydroxyl) and hydrophobic (carbon chain).
In summary, it is an anionic surfactant that promotes particle dispersion (Ouyang; Qiu; Chen, 2006). For water reducers/plasticizers, there are two main dispersing mechanisms: electrostatic repulsion and steric hindrance. The predominant mechanism depends on the plasticizer type. In the case of Lignosulfonate, both mechanisms disperse cement particles (Colombo et al., 2017).
Furthermore, previous studies indicate that plasticizers can cause retardation of the setting time of cement paste. In the literature, various mechanisms are suggested to explain this behavior, including calcium complexation, nucleation poisoning of hydrates, surface adsorption on anhydrous cement particles, and the presence of sugars in the plasticizer. However, in the case of lignosulfonate water reducer, the sugars (such as glucose and sucrose) contained in lignin seem to have a significant contribution to longer setting times in cement (Colombo et al., 2017; Arel; Aydin, 2017). Danner et al. (2014) observed that in the presence of LS, the hydration of C3S and C3A was retarded and influenced the solubility of the sulfate phase, accelerating the initial dissolution of gypsum resulting in the acceleration of the initial ettringite formation.
In this context, this work aims to analyze three types of cement with novel compositions of calcium sulfate with different proportions of untreated phosphogypsum (PG), natural gypsum (NG), and phosphogypsum neutralized with 5% solid hydrated lime (NPG) in the presence of lignosulfonate-based admixture regarding the hydration kinetics and rheological behavior of cement pastes. This study was investigated through an experimental program where fresh cement and harden properties were assessed with and without admixture presence. The main contribution of this study lies in bridging the current gap concerning the integration of PG into Portland cement as a set regulator in the presence of admixture. This area remains relatively underexplored in the existing literature. The study tries to overcome the two main challenges related to using PG in cement: delays in setting and reduction of initial strengths. The study focuses on using different proportions of PG combined with NG and calcium hydroxide treatment. Additionally, the study provides perspectives for the future development of industrially viable solutions.
Methods
This study’s experimental research was divided into three main steps. The first stage consisted of raw materials’ physical-chemical, mineralogical, and thermal characterization. Secondly, the cement’s fresh and hardened properties were assessed without admixture presence, such as initial and final setting and compressive strength. Finally, the hydration and rheological behavior of the cement in the admixture presence was evaluated.
The following materials were used in this research: three novel combinations of calcium sulfate sources (SU I, SU II, and SU III), described in Table 1, and three compositions of Portland cement (CEM I, CEM II, and CEM III), showed Table 2. The SUs were produced using combinations of raw phosphogypsum (PG), natural gypsum (NG), and neutralized phosphogypsum (NPG) with hydrated lime (CH III). Each SU type was used as a calcium sulfate source to regulate the cement setting. The CEMs were produced with SU, clinker, and limestone. Also, calcium sulfate content was adjusted according to the cement sulfate demand described in C563-18 (ASTM, 2018) and complied with the 4.5 % SO3 limit requested by NBR 16697 (ABNT, 2018a). Finally, the cement was milled using 600 g of grinding aid per ton.
Additionally, a water-reducing admixture type 1 (AD 01), based on lignosulfonate, was used in this study at a dosage of 0.6% by mass of binder. The admixture had a specific gravity of 1.100 - 1.140 g/cm³, with dark brown coloration. The choice of the lignosulfonate admixture in this study is related to its everyday use in Brazilian concrete manufacturing companies.
The chemical composition of raw materials was evaluated using fused pellets in the X-ray fluorescence spectrometer Zetium Cement edition, Panalytical, with 400-4000 cm-1 wavelength. SU samples’ hydrogen potential (pH) was carried out following a 1:10 solid-to-water ratio and NBR 7353 (ABNT, 2019a). A mPA210 pH meter, MS TECHNOPON Instrumentation, was used to perform the test. Inductively coupled plasma-optical emission spectrometry (ICP-OES) was performed to measure SU samples soluble P2O5. An Agilent technologies ICP-OES was used following Costa et al. (2022) procedure to carry out this test. The admixture infrared spectra were obtained using Shimadzu Prestige-21 with 4.000 to 400 cm-1 wavelengths.
Mineralogical characterization of novel SU was executed through X-ray diffraction technique (XRD) using a Bruker D8 Advance diffractometer equipped with a CuK radiation source, a Ni filter, and a 0.020° step size. The 2θ scan range was set from 5° to 65°, with a scan speed of 1.7°/min, and the sample rotated at 30 rpm speed.
The thermogravimetric analysis (TGA) of SUs was conducted using the SDT Q600 TA Instruments. The samples were analyzed in a 110 μL alumina crucible, with a heating rate of 10 °C/min until it reached 1000 °C.
Particle size distribution and specific cement surface were determined through laser granulometry, and the Blaine fineness determination was determined using the air permeability method. Blaine was executed following NBR 16372 (ABNT, 2015), and laser granulometry was carried out on a CILAS 1180 laser granulometer, with isopropyl alcohol as the solvent, and scattered using ultrasound treatment for 60 s. Also, PG and NG morphology were investigated through scanning electron microscopy (SEM) by secondary electrons in an SEM JSM 6060 with a voltage of 6kV. The samples used were in powder form and were dispersed onto a carbon tape attached to the stub and metalized with gold.
The isothermal conduction calorimetry was performed using a TA Instruments 8-channel TAM AIR isothermal calorimeter at a constant temperature of 25 ± 0.05 ºC for 72 h. The cement was mixed with deionized water in an automatic mixer at 10.000 rpm for 2 min. Cement pastes were produced with a water-to-cement ratio of 0.45. Reference ampoules used water with a mass adjusted to have a calorific value compatible with the cement paste samples. Additionally, the cement and mixing water were stored previously for 24 hours in the calorimeter’s room to equalize their temperature to 25 ºC. The results of heat flow and heat release (mW/g) were normalized by the mass of the paste added to the ampoule.
The initial (IP) and final (FP) setting times were determined according to NBR 16607 (ABNT, 2018b), and the compressive strength of cement at ages of 1, 3, and 28 days without lignosulfonate admixture was assessed following NBR 7215 (ABNT, 2019b), using six specimens by age. The standard deviation followed the normative standard.
The rheology behavior of the CEM in the presence and absence of the admixture was assessed. The rheology parameters were determined through rotational rheometry using a Haake MARS 40 rheometer. A cylinder concentric geometry was used in the test (20 mm diameter and 11 mm height). Firstly, a pre-shear stress of 100 s-1 is applied during 60s. Secondly, a shear rate is increased from 0.1 to 100 s-1 and reduced from 0.1 s-1 to obtain flow curves. The dynamic yield stress (Ʈ0) was determined using the descendent part of the yield curve Herschel-Bulkley model (H-B). The plastic equivalent viscosity (µeq) was calculated according to De Larrard, Ferraris, and Sedran, 1998). The cement paste preparation followed the isothermal calorimetry procedure.
For cement paste analysis, the thermogravimetric equipment was a METLER TOLEDO TGA2. The cement paste hydration was stopped at 6h, 24h, and 48h with isopropyl alcohol, according to Scrivener, Snellings and Lothenbach (2016). The samples were in powder form (#75µm). The combined water measured the difference between the weight losses of samples dried to 105 and at 1000 °C, Equation 1; portlandite was calculated from the difference in weight loss between 400 to 500 °C according to Equation 2. The samples were analyzed in a 110 μL alumina crucible, with a heating rate of 20°C/min until it reached 1000 °C.
Where:
WL(105-1000 °C): weight loss between 105 and 1000 °C;
WL(400-500 °C): weight loss between 400 and 500 °C;
mCa(OH)2: 74g/mol (molecular mass of Ca(OH)2); and
mH2O: 18g/mol (molecular mass of H2O).
Raman spectroscopy was performed to analyze the hydration products. The cement paste hydration was stopped at 6h, 24h, and 48h with isopropyl alcohol, according to Scrivener, Snellings and Lothenbach (2016). The samples were in powder form (#75µm). The Raman spectroscopy equipment was a Renishaw inVia with a 200-4000 cm-1 wavelength and a 532 nm laser.
Results and discussion
Physical-chemical, mineralogical, and thermal characterization of raw materials
Table 3 displays information on the chemical composition, mineralogy, and pH of the materials utilized in the study. The novel compositions of calcium sulfate sources (SU I, II, and III) employed in the studied cements (CEM I, II, and III) were produced using two distinct pH calcium sulfate sources: an acidic pH PG (3.05) with approximately 1% P2O5; and a basic pH NG (8.66) without detectable phosphorus, as outlined in Table 3. In this table, the clinkers (CL I, II, and III) used in the cement compositions (CEM I, II, and III) have similar chemical and mineralogical compositions. Also, when analyzing the P content of SU, Table 4 shows that SU I (70% NG and 30%PG) had no detectable P content. On the other hand, SU II (50% NG and 50 % NPG) and SU III (100% NPG) showed a P higher content of approximately 0.54 and 0.64 %, respectively. This difference is associated with the higher PG content in these SU compositions (50% and 100%). Nevertheless, although SU III exhibited a higher P content, the difference compared to SU II was only 0.1%. Furthermore, the P2O5 soluble in SU I showed a 12% and 6% lower ppm content than in SU II and III. Also, it is evident that compared to untreated PG, which has an acidic pH (3.05), SU I, II, and III exhibited an alkaline pH, Table 5. A non-acid calcium sulfate source is essential to maintain the alkaline environment generated by cement hydration.
Chemical composition in oxides (%) and pH of PG and NG used in sulfate source formulations, and chemical composition in oxides (%) with C3S* * calculated by Bogue (ASTM, 2007). (%) and C3A* * calculated by Bogue (ASTM, 2007). (%) contents of the clinkers used to produce the cements
Figure 1(a) shows TG and DTG of SU I, II, and III, displaying a mass loss in the following temperature ranges: 100-200 °C, 400-450 °C, and 600-750 °C. The first mass loss range is related to the dehydration of gypsum of CaSO4·2H2O into CaSO4. The second mass loss range is only observed in SU II and III, more evidently in later. The mass loss at 400-450 °C and 600-750 °C is probably related to the decomposition of portlandite (Ca(OH)2) and decarbonation of calcium carbonate (CaCO3) from the hydrated lime used in the neutralization process. The total weight loss increases from 1.6% to 9.3% when comparing SU I to SU II and SU III, respectively.
Moreover, Figure 2 presents the mineralogical composition of SU through X-ray diffraction (XRD). As expected, all calcium sulfate sources are composed of CaSO4·2H2O. CaSO4. SiO2 was also detected.
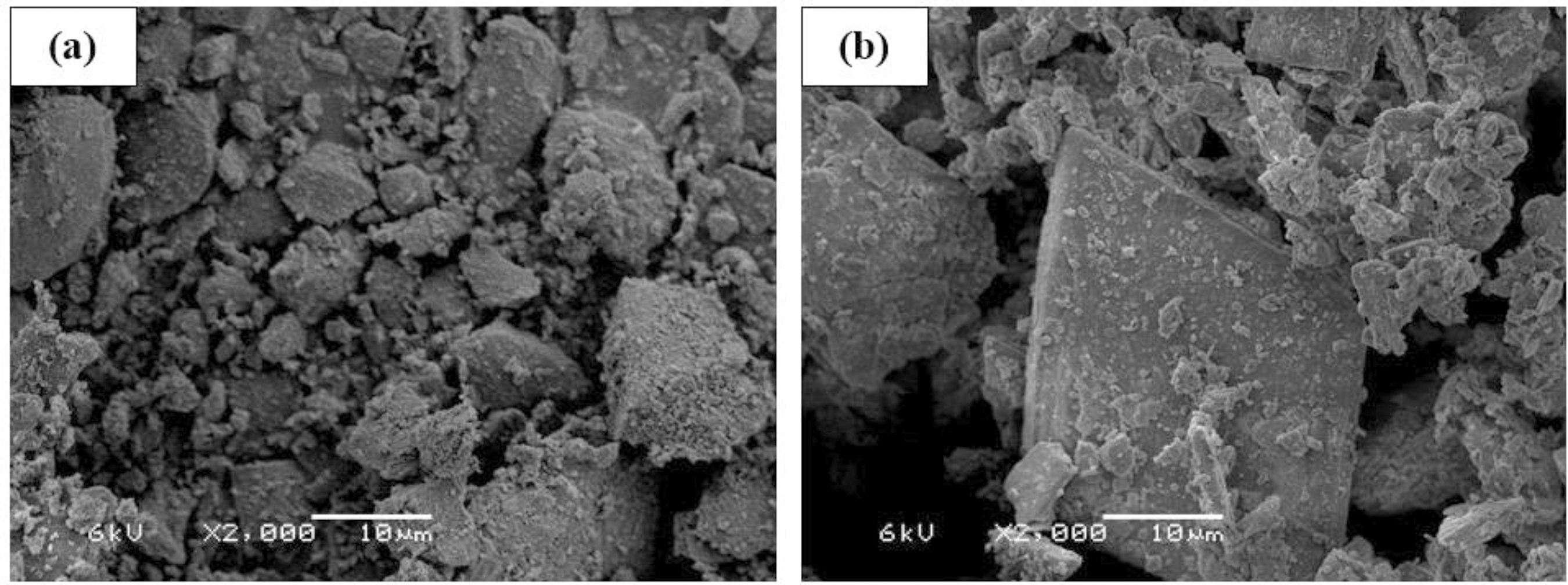
Regarding physical characteristics, Table 6 shows CEM’s cement particle size and fineness. The results indicate that CEM have similar particle size distribution but slightly differ in fineness. The mean diameter (Dv average) is around 14-15µm and the fineness of CEM I is 0.29 and 2.38% lower than CEM II and III, respectively. This variation in particle size and fineness does not affect the result significantly. Also, Figure 3 presents NG (a) and PG (b) morphology by SEM through secondary electrons. From the images, NG shows particles with a granular appearance, while in PG, particles with a prismatic and lamellar appearance can be seen.
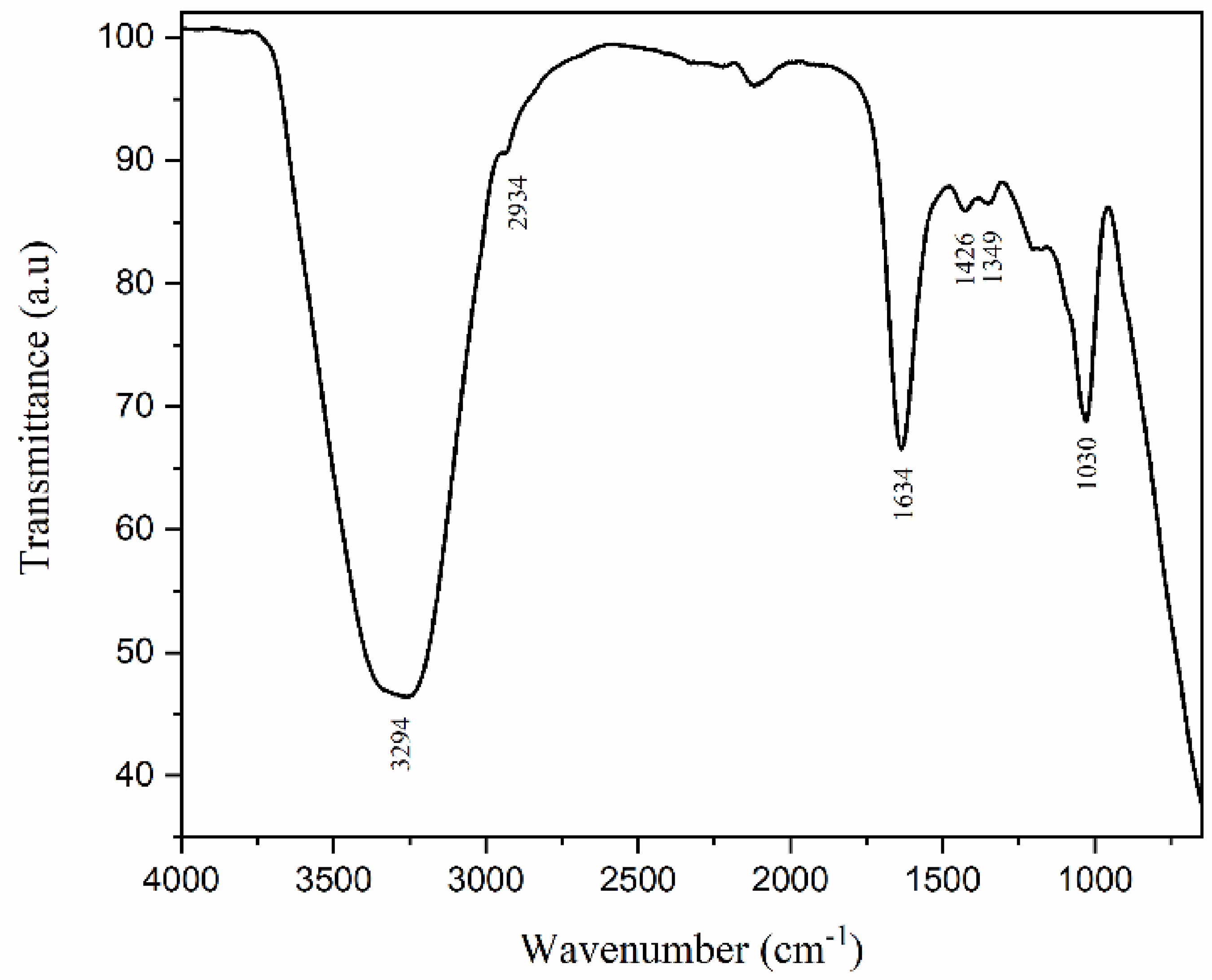
Figure 4 shows the chemical admixture FTIR spectra. A broad band at 3294 cm-1 attributed to the O-H bond of water is identified. The absorption band at 2934 cm-1 is characteristic of the stretching vibration of the C-H bond in aliphatic groups. The bands at 1426 and 1349 cm-1 are assigned to the C-H bending in alkanes, and the band at 1030 cm-1 corresponds to the SO3 group (Palacios; Puertas, 2004; 2005).
Cement fresh and hardened properties
Table 7 contains characterizations of cement regarding setting times and compressive strength at different ages. CEM I, II, and III show similar strength gain patterns at 3 and 28 days. In contrast, CEM I exhibit approximately 15% and 49% higher strengths than CEM II and III, respectively. Additionally, CEM III shows setting times 24% and 19% higher than CEM I and II, respectively. These results suggest that SU I and II outperform cements with SU III in terms of the evaluated properties. The latter exhibits more pronounced delays in setting and strength gain in 1 day. However, by 3 days, this difference in strength is reduced. This scenario highlights that the treatment with 5% solid hydrated lime in the PG was insufficient to utilize it as a calcium sulfate source in cement fully. This behavior may be associated with soluble phosphates and fluorides reacting with calcium ions, causing the precipitation of CaF2 and Ca3(PO4)2. According to some authors, this phenomenon delays the dissolution and hydration of the cement (Bénard et al., 2005).
Initial (IP) and Final (FP) setting times and compressive strength at ages of 1 (R1), 3 (R3), and 28 (R28) days without lignosulfonate admixture
Hydration and rheological behavior of cement in the presence of admixture
The samples CEM I-0 and CEM II-0 (without AD_01) exhibit similar behaviors in terms of heat flow and cumulative heat (Figure 5(a) and 5(b)). The curves from CEM I-0 and CEM II-0 curves almost overlap. However, the CEM III-0 sample shows delays in reactions of approximately 1.37 h. This result supports the trend observed in the setting times (IP and FP) tests (Table 7), where CEM III showed longer setting times. Also, the admixture causes delays in reactions, leading to an extension in the initial induction period and consequently delaying the acceleration phase and the attainment of the peak. In the CEM III-01 cement (with AD_01), the retarded reaction is even more pronounced.
Heat flow curves (a) and cumulative heat (b) of the cement paste CEM I, II, and III with and without chemical admixture
Additionally, a reduction in the heat released throughout the process was observed. This behavior was also followed by Andrade Neto et al. (2021), where the plasticizer admixture delayed hydration, prolonged induction, delayed acceleration periods, and reduced total heat release. This difference could be explained by the sugars in the plasticizers, which are lignosulfonate, that adsorb in the anhydrous phases and cement hydrates.
Also, in the Andrade Neto et al. (2021) study, the cement pastes with PG treated with calcium hydroxide reduced the phosphorus and fluorine content and the delay in hydration compared to the samples with untreated PG. However, a prolongation of the induction period of the treated PG sample was still identified compared to the cement paste with NG in the author’s investigation.
Regarding the cumulated heat presented in Figure 7, the cements without admixture showed a higher cumulative heat at 24h, as expected, compared to the cements with the admixture at 24h, approximately 2.5 times higher. When comparing the types of cement without admixture, CEM I indicated a cumulative heat of approximately 0.71 and 5.38% higher than CEM II and CEM III, respectively. In contrast, when comparing CEM in the presence of admixture, the cumulative heat at 24h of CEM I was approximately 9% lower than CEM II and 192% higher than CEM III. Holanda, Schmidt, and Quarcioni (2017) indicated in a previous study that the increase in phosphorus content delays the induction period and decreases the reaction rates of the chemical reactions. In summary, the observed trend followed this sequence: CEM III - 01 (31.13 J/g) < CEM I -01 (59.78 J/g) < CEM II – 01 (65,83 J/g) < CEM III (123.28 J/g) < CEM II (128.99) < CEM I (129.91). This trend shows the lignosulfonate admixture significantly impacted the cumulative heat at 24h. Without an admixture scenario, the difference values between the samples were less than 10%. Also, Holanda, Schmidt, and Quarcioni (2017) suggest the existence of a critical phosphorus contamination concentration in the cement paste with or without admixture, which is 1.13% in the P2O5 form on calcium sulfate. Also, the range between 0.83 and 1.64% are critical concentrations where the delay in hydration is more evident. According to the authors, beyond this range, a saturation of calcium phosphate precipitation occurs on the cement grains, making the alterations and delays negligible.
However, when analyzing the 72h cumulative heat, only a slight difference between the CEMs with and without admixture is observed. The observed trend for these samples is CEM I (199.81J/g) > CEM II (198.86 J/g) > CEM III (197.34 J/g) > CEM I – 01 (194.03 J/g) > CEM II – 01 (195.43 J/g) > CEM III – 01 (190.96 J/g). CEM I (199.8 J/g), which has the highest cumulative heat at 72h, is only approximately 4.6 % higher than CEM III – 01 (190.96 J/g), which has the lowest heat at 48h. Thus, these results suggest that the novel SU and admixture effect was mainly in the first 24h, while at 72h, no significant difference was identified between the samples. Holanda, Schmidt, and Quarcioni (2017), when analyzing portlandite formation through TGA in cement with PG, various phosphorus content, and superplasticizer, observed no significant difference between the samples after 72h. This result corroborates the finding where similar cumulative heat is obtained after 72h. The cumulative heat and portlandite formation are indications of the reaction rate.
The retardation effect observed in the samples with the chemical admixture agrees with delays in the setting time observed in previous studies (Arel; Aydin, 2017; Ouyang; Qiu; Chen, 2006). The literature suggests that the glucose and sucrose contained in the substance significantly contribute to longer setting times in cement hydration (Colombo et al., 2017; Arel; Aydin, 2017). Nevertheless, phosphorus and fluorine are believed to accentuate this behavior further, potentially rendering it impractical to utilize LS as a water reducer in cement compounded with PG, which serves as a setting regulator (source of calcium sulfate). Also, a more efficient neutralization process for PG could be an alternative. In terms of comparison of the heat flow and cumulative heat curves between CEM I and II with and without admixture, these curves were very similar. The result suggests that these two novel calcium sulfate sources could be used in exchange for each other as solutions to the cement industry. In contrast, CEM III showed a much lower performance.
Figure 8 shows the TG and DTG profiles of hydrated cement pastes (CEM I, II, and III) at different ages: 3, 6, 24, and 48 hours. The mass loss occurs in three distinct temperature ranges: 100-150 °C, 400-450 °C, and 600-750 °C.
100-150 °C: At 3 and 6 hours, this mass loss is likely associated with the dehydration of gypsum (CaSO4·2H2O), which typically loses its water in two steps: at around 100-140 °C to form hemihydrate (CaSO4·0.5H2O) and at 140-150 °C to form anhydrite (CaSO4). Generally, these two water loss peaks overlap significantly. At 24 and 48 hours, the mass loss is likely due to the dehydration of calcium silicate hydrate (C-S-H) and ettringite. 400-450 °C: This mass loss is attributed to the decomposition of portlandite (Ca(OH)2). 600-750 °C: The mass loss in this range is probably due to the decarbonation of calcium carbonate (CaCO3) originated from the filler used in the cement and minor carbonation of the sample during storage.
Figure 9 exhibits the portlandite content of hydrated pastes from CEM I, II, and III at 3, 6, 24, and 48h. The results show that the cements have lower portlandite content when the admixture is added to the cement paste. This result corroborates the behavior observed in the isothermal calorimetry, where the cement pastes with admixture had a more delayed reaction, consequently impacting the portlandite formation. At 24h, CEM I, II, and III have approximately 85%, 82%, and 84% lower portlandite content when the admixture is used. When comparing the cements without admixture, the highest portlandite content, at 24h, is obtained to CEM II (12%) while the lowest to CEM I (9.23%).
Portlandite content (%) of hydrated pastes: CEM I (a), CEM II (b), and CEM III (c) at 3, 6, 24 and 48h
Figure 10 shows the Raman spectrum comparison of CEM I II and III hydrated cement pastes with (AD1) and without (AD0) admixture at 6 and 24h. Raman spectroscopy examines molecular vibrations and structural changes by analyzing how light scatters when interacting with a material. This method detects energy exchange between incident photons and molecular bonds, emitting scattered light with varied energy levels for analysis. Different material phases or components display unique Raman spectra due to molecular vibrations or crystal structure differences. As materials undergo hydration, phase transitions occur, altering composition and structure and affecting bond lengths, strengths, and molecular interactions. These transitions are reflected in Raman spectra through changes in peak positions, intensities, or line widths, with increased peak intensity indicating molecular species accumulation or enhancement (Mario et al., 2023).
Intensity comparison of the Raman shift (cm-1) from the hydrated cement pastes at 6h and 24h
In Figure 11, it is possible to detect a peak in 518 cm-1 and others between 900-100 cm-1, but since there is a big intensity gap between the peaks, it is hard to identify them. Thus, two breaks in the Y-axis, in Figure 11, were made to improve resolution from 0.04 to 0.9 and 9 to 1.8. Ettringite (Aft), gypsum, and monosulfate are suggested to happen in previous studies around 987-992, and 1008-1020, 979-984 cm-1 respectively (Black et al., 2006; Garg; Wang; Martin, 2013; Liu; Sun; Qi, 2015; Quiroz-Portillo et al., 2023; Renaudin et al., 2007; Sahu; Exline; Nelson, 2002). These sulfates have very similar characteristic shifts. Liu, Sun, and Qi (2015) identified three peaks at 983, 991, and 1005 cm-1, which were assigned to monosulfate, ettringite, and gypsum. Additionally, the peak at 518 cm-1 could be associated to the , which could indicate belite (C2S) and alite (C3S) (Masmoudi et al., 2017; Zhang et al., 2023).
Furthermore, since the intensity of the Raman peak indicates the concentration of material, Figure 10 was plotted with the actual intensity of samples. The results show that peak intensity located in 518 cm-1 related to is higher in all samples where no admixture was used at 6h. This trend suggests a higher silicate content in these samples. However, this behavior does not comply with the assumption that the cement pastes with admixture have a delayed reaction and, consequently, a lower silicates consumption at 6h, which means a higher concentration of silicates in the samples with admixture than those without admixture. In the 24h hydration, the Raman spectra intensity seems to have a similar height, which would mean no significant difference between the samples in terms of concentration.
Rotational rheometer
Figure 12 presents the rheological properties of the evaluated cement pastes. In Figure 9(a), the descending flow curves are shown. Noticeably, the higher content of treated phosphogypsum reduced the pastes’ shear stress without using lignosulfonate admixture. It is worth noting that a good fit of the shear curve was obtained using the H-B model (R2 > 0.996), where all pastes showed pseudoplastic parameter values different from 1. Thus, a linear fit, such as the Bingham model, is inadequate to describe this rheological behavior.
Parameters of (a) shear stress, (b) dynamic yield stress, (c) equivalent viscosity, and (d) hysteresis area of cement pastes
Regarding Figures 12(b) and 12(c), the lignosulfonate admixture addition reduced dynamic yield stress and equivalent viscosity values, providing greater fluidity to the mixtures. This behavior was expected since the polymer acts through electrostatic repulsion (Arel; Aydin, 2017). Additionally, it can be observed that cement CEM III_100 exhibited greater fluidity, with lower yield stress and plastic viscosity. This tendency likely occurred due to the delay caused by the purified phosphogypsum in the cement hydration, as Andrade Neto et al. (2021) reported.
The presence of contaminants (e.g., fluorine and phosphorus) in phosphogypsum delays the formation of hydrated phases, especially ettringite, in the early hours. With its high surface area, ettringite increases dynamic yield stress and paste viscosity. Therefore, the likely more significant initial ettringite formation in CEM I and II without admixture justifies the lower fluidity observed than cement CEM III (García-Maté et al., 2015). Navarrete et al. (2022) reported that the increase in viscosity is linked to two main factors: the number of contact points due to particle density and the interparticle force resulting from the roughness of cementitious materials.
Vikan et al. (2007) observed that for the same type of clinker or clinker with similar mineralogical composition (as is the case in this study), the paste fluidity increases as the Blaine surface area of the cement decreases. However, this behavior was the opposite in this investigation, which indicated that the higher the Blaine surface area, the greater the paste fluidity.
Through the stress versus shear rate graph, it was possible to calculate the hysteresis area (difference in stress between the acceleration curve and the deceleration curve), which represents the energy required to break down the clusters in the evaluated pastes, indicating the homogeneity of the mixtures according to Figures 12 (d). It can be noted that the obtained values are consistent since, at high shear stresses, without the presence of admixtures, solid segregation does not occur, maintaining the behavior pattern of Figures 12 (b) and (c). On the other hand, in the presence of lignosulfonate, there is a reduction in the hysteresis area due to the dispersion of cement particles in the system, preventing the formation of clusters; these results are consistent with Cheung, Roberts, and Liu (2018).
Conclusions
The findings indicate that utilizing a composition of 100% lime-treated phosphogypsum (NPG), referred to as SU III (100% NPG), as a calcium sulfate source in cement results in inferior performance compared to cement formulations incorporating combinations of 70% natural gypsum (NG) and 30% untreated phosphogypsum (PG), referred as SU I (70% NG and 30% PG), or 50% NG and 50% NPG, referred as SU II (50% NG and 50% NPG). This disparity is evident in lower early strengths (1 day) and extended setting times, indicating delayed hydration reactions.
Moreover, the inclusion of lignosulfonate admixtures exacerbates these differences among the samples. The sugars (glucose and sucrose) in the admixture are suggested to contribute to prolonged setting times during cement hydration. Additionally, phosphorus and fluorine are believed to accentuate this behavior further, potentially rendering lignosulfonate as a water reducer impractical in cement containing PG (calcium sulfate source) as a setting regulator.
Exploring more effective neutralization alternatives to PG could be a viable solution. However, cost and secondary waste generation must be carefully considered when evaluating options. The feasibility of the chosen treatment method on an industrial scale, associated costs, and the management of secondary wastes generated during the process demand thorough consideration. The cost of treating PG impurities should not be excessively high, as this could render PG impractical as a setting regulator in cement production.
It was also observed that cement produced with SU II and III, referred to as CEM II and III, exhibited lower yield stress levels in rheological parameters than CEM I (70% NG and 30% PG). This observation may be attributed to a potential delay induced by phosphogypsum, particularly in proportions exceeding 30%, even when treated with lime.
References
- ABRIL, J. M.; GARCÍA-TENORIO, R.; MANJÓN, G. Extensive radioactive characterization of a phosphogypsum stack in SW Spain: 226Ra, 238U, 210Po concentrations and 222Rn exhalation rate. Journal of Hazardous Materials, v. 164, n. 2–3, p. 790–797, 2009.
- AKFAS, F. et al. Exploring the potential reuse of phosphogypsum: a waste or a resource? Science of the Total Environment, v. 908, p. 168196, 2024.
- AMERICAN SOCIETY FOR TESTING AND MATERIALS. C563-18: approximation of optimum SO3 in hydraulic cement. West Conshohocken, 2018.
- AMERICAN SOCIETY FOR TESTING AND MATERIALS. C150-07: standard specification for Portland cement. West Conshohocken, 2007.
- ANDRADE NETO, J. S. et al. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Construction and Building Materials, v. 299, p. 123935, 2021.
- AREL, H. Ş.; AYDIN, E. Effects of Ca-, Mg-, K-, and Na-lignosulfonates on the behavior of fresh concrete. Construction and Building Materials, v. 157, p. 1084–1091, 2017.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16372: cimento Portland e outros materiais em pó: determinação da finura pelo método de permeabilidade ao ar (Método Blaine). Rio de Janeiro, 2015.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16607: cimento Portland: determinação dos tempos de pega. Rio de Janeiro, 2018b.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 16697: cimento Portland: requisitos. Rio de Janeiro, 2018a.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7215: cimento Portland: determinação da resistência à compressão de corpos de provas cilíndricos. Rio de Janeiro, 2019b.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 7353: soluções aquosas: determinação do pH com eletrodos de vidro. Rio de Janeiro, 2019a.
- BÉNARD, P. et al. Hydration process and rheological properties of cement pastes modified by orthophosphate addition. Journal of the European Ceramic Society, v. 25, n. 11, p. 1877–1883, 2005.
- BÉNARD, P. et al. Influence of orthophosphate ions on the dissolution of tricalcium silicate. Cement and Concrete Research, v. 38, n. 10, p. 1137–1141, 2008.
- BLACK, L. et al. Hydration of tricalcium aluminate (C3A) in the presence and absence of gypsum: studied by Raman spectroscopy and X-ray diffraction. Journal of Materials Chemistry, v. 16, n. 13, p. 1263–1272, 2006.
- CAI, Q. et al. Efficient removal of phosphate impurities in waste phosphogypsum for the production of cement. Science of the Total Environment, v. 780, 2021.
- CANUT, M. M. C. et al. Microstructural analyses of phosphogypsum generated by Brazilian fertilizer industries. Materials Characterization, v. 59, n. 4, p. 365–373, 2008.
- CAO, W. et al. A facile approach for large-scale recovery of phosphogypsum: an insight from its performance. Construction and Building Materials, v. 309, p. 125190, 2021.
- CHEN, X. et al. Effect of neutralization on the setting and hardening characters of hemihydrate phosphogypsum plaster. Construction and Building Materials, v. 190, p. 53–64, 2018.
- CHEN, X. et al. Study on physical and chemical characteristics of β-hemihydrate phosphogypsum. Case Studies in Construction Materials, v. 17, p. e01461, 2022.
- CHEUNG, J.; ROBERTS, L.; LIU, J. Admixtures and sustainability. Cement and Concrete Research, v. 114, p. 79-89, 2018.
- COLOMBO, A. et al. On the effect of calcium lignosulfonate on the rheology and setting time of cement paste. Cement and Concrete Research, v. 100, p. 435-444, 2017.
- COSTA, A. R. D.; GONÇALVES, J. P. Milling parameters and solid waste characterization to use as supplementary cementitious materials. Ambiente Construído, Porto Alegre, v. 22, n. 4, p. 35–48, out./dez. 2022.
- COSTA, R. P. et al. Effect of soluble phosphate, fluoride, and pH in Brazilian phosphogypsum used as setting retarder on Portland cement hydration. Case Studies in Construction Materials, v. 17, p. e01413, 2022.
- CUI, Y. et al. Effects of Ca(OH)2 on the early hydration, macro-performance and environmental risks of the calcined phosphogypsum. Construction and Building Materials, v. 324, p. 126590, 2022.
- DANNER, T. et al. Phase changes during the early hydration of Portland cement with Ca-lignosulfonates. Cement and Concrete Research, v. 69, p. 50-60, 2014.
- DE LARRARD, F.; FERRARIS, C. F.; SEDRAN, T. Fresh concrete: a HerscheI-Bulkley material. Materials and Structures, v. 31, p. 494–498, 1998.
- GARCÍA-MATÉ, M. et al. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cement and Concrete Composites, v. 55, p. 53–61, 2015.
- GARG, N.; WANG, K.; MARTIN, S. W. A Raman spectroscopic study of the evolution of sulfates and hydroxides in cement-fly ash pastes. Cement and Concrete Research, v. 53, p. 91–103, 2013.
- GUERRERO‐MÁRQUEZ, J. L. et al. Occupational, public and environmental radiological impact caused by the phosphoric acid industry: the case of Huelva (Spain). In: PHOSPHORIC acid industry: problems and solutions. London: InTech, 2017.
- HOLANDA, F. do C.; SCHMIDT, H.; QUARCIONI, V. A. Influence of phosphorus from phosphogypsum on the initial hydration of Portland cement in the presence of superplasticizers. Cement and Concrete Composites, v. 83, p. 384–393, 2017.
- JIA, R.; WANG, Q.; LUO, T. Reuse of phosphogypsum as hemihydrate gypsum: The negative effect and content control of H3PO4 Resources, Conservation and Recycling, v. 174, p. 105830, 2021.
- LI, X.; ZHANG, Q. Dehydration behaviour and impurity change of phosphogypsum during calcination. Construction and Building Materials, v. 311, p. 125328, 2021.
- LIU, F.; SUN, Z.; QI, C. Raman spectroscopy study on the hydration behaviors of Portland cement pastes during setting. Journal of Materials in Civil Engineering, v. 27, n. 8, 2015.
- LIU, S.; WANG, L.; YU, B. Effect of modified phosphogypsum on the hydration properties of the phosphogypsum-based supersulfated cement. Construction and Building Materials, v. 214, p. 9–16, 2019.
- LV, X.; XIANG, L. Investigating the novel process for thorough removal of eutectic phosphate impurities from phosphogypsum. Journal of Materials Research and Technologies, v. 24, p. 5980-5990, 2023.
- MARIO, M. et al. Raman spectroscopy: part one. In: NON-DESTRUCTIVE material characterization methods. New York: Elsevier, 2023.
- MASMOUDI, R. et al. In situ Raman studies on cement paste prepared with natural pozzolanic volcanic ash and Ordinary Portland Cement. Construction and Building Materials, v. 148, p. 444–454, 2017.
- NAVARRETE, I. et al. Effect of supplementary cementitious materials on viscosity of cement-based pastes. Cement and Concrete Research, v. 151, 2022.
- OUYANG, X.; QIU, X.; CHEN, P. Physicochemical characterization of calcium lignosulfonate: a potentially useful water reducer. Physicochemical and Engineering Aspects, v. 282, n. 283, p. 489–497, 2006.
- PALACIOS, M.; PUERTAS, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cement and Concrete Research, v. 35, n. 7, p. 1358–1367, 2005.
- PALACIOS, M.; PUERTAS, F. Stability of superplasticizers and shrinkage-reducing admixtures in high basic media. Materiales de Construcción, v. 54, n. 276, p. 65–86, 2004.
- PALLA, S. et al Solar thermal treatment of phosphogypsum and its impact on the mineralogical modification for effective utilization in cement production. Journal of Building Engineering, v. 51, 2022.
- QI, H. et al Influence of fluoride ion on the performance of PCE in hemihydrate gypsum pastes. Journal of Building Engineering, v. 46, 2022.
- QIN, X. et al Resource utilization and development of phosphogypsum-based materials in civil engineering. Journal of Cleaner Production, v. 387, p. 135858, 2023.
- QUIROZ-PORTILLO, D. et al Study of the Hydration Mechanism of Portland cement with raman spectroscopy applying CO2 laser radiation. Journal of Spectroscopy, v. 2023, p. 1–7, 2023.
- RASHAD, A. M. Phosphogypsum as a construction material. Journal of Cleaner Production, v. 166, p. 732–743, 2017.
- RENAUDIN, G. et al. A raman study of the sulfated cement hydrates: ettringite and monosulfoaluminate. Journal of Advanced Concrete Technology, v. 5, n. 3, p. 299-312, 2007.
- ROSALES, J. et al Treated phosphogypsum as an alternative set regulator and mineral addition in cement production. Journal of Cleaner Production, n. 244, p. 118752, 2019.
- SAHU, S.; EXLINE, D. L.; NELSON, M. P. Identification of thaumasite in concrete by Raman chemical imaging. Cement and Concrete Composites, v. 24, p. 347–350, 2002.
- SCRIVENER, K.; SNELLINGS, R.; LOTHENBACH, B. A practical guide to microstructural analysis of cementitious materials. Boca Raton: CRC Press, 2016.
- SILVA, L. F. O. et al A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere, v. 286, p. 131513, 2022.
- SINGH, M. Treating waste phosphogypsum for cement and plaster manufacture. Cement and Concrete Composites, v. 32, p. 1033–1038, 2002.
- TABIKN, A. A.; MILLER, F. M. The natutre of phosphogypsum impurities and their influence on cement hydration. Cement and Concrete Research, v. 1, p. 663–678, 1971.
- TAYIBI, H. et al. Environmental impact and management of phosphogypsum. Journal of Environmental Management, v. 90, n. 8, p. 2377–2386, 2009.
- VIKAN, H. et al. Correlating cement characteristics with rheology of paste. Cement and Concrete Research, v. 37, n. 11, p. 1502–1511, 2007.
- XU, J. et al. Mechanical properties and soluble phosphorus solidification mechanism of a novel high amount phosphogypsum-based mortar. Construction and Building Materials, v. 394, 2023.
- ZAN, C. et al. Evaluation method for thermal processing of phosphoric acid with waste heat recovery. Energy, v 31, 2791–2804, 2006.
- ZHANG, B. et al In situ monitoring of the hydration of calcium silicate minerals in cement with a remote fiber-optic Raman probe. Cement and Concrete Composites, v. 142, 2023.
- ZHANG, J. et al Effect of soluble P2O5 form on the hydration and hardening of hemihydrate phosphogypsum. Advances in Materials Science and Engineering, v. 2022, 2022.
Edited by
-
Editor:
Enedir Ghisi
-
Editora de seção:
Edna Possan
Publication Dates
-
Publication in this collection
17 Mar 2025 -
Date of issue
Jan-Dec 2025
History
-
Received
03 May 2024 -
Accepted
20 July 2024

 Effect of novel calcium sulfate compositions and lignosulfonate admixture on cement hydration and rheology
Effect of novel calcium sulfate compositions and lignosulfonate admixture on cement hydration and rheology