Abstract
Background: Swallowwort (Cynanchum acutum subsp. sibiricum) poses significant challenges in cotton fields and orchards across northwest China.
Objective: Laboratory and greenhouse experiments were conducted to investigate the impact of various abiotic factors on seed germination of swallowwort.
Methods: Seed germination and seedling emergence of freshly harvested seed (FS) and seed stored for six months (SS) were investigated under different temperatures, osmotic potentials, saline stresses, and burial depths.
Results: The optimal germination temperature of FS was 30 to 35 °C, while that for SS was extended to 15 to 35 °C. Freshly harvested seeds germinated under alternating temperature regimes from 20/10 to 45/35 °C, but seed germination could reach to 60% at 15/5 °C after six months of storage. No germination of FS was observed at ≤ -1.0 MPa. However, seed germination remained at 44.6% at -1.0 MPa after storage. Saline stress reduced seed germination, with no germination observed at NaCl concentrations of ≥ 400 mM for the FS or ≥ 500 mM for the SS. However, pH values ranging from 5 to 10 had no significant impact on seed germination. The maximum seedling emergence (71%) was observed at the soil surface and no emergence at 10-cm burial depth.
Conclusions: Six months of storage enhanced seed tolerance to sub-optimal temperature, drought and salt stress conditions, comparing with FS. This made swallowwort more adaptable to harsh environments such as drought and salinity. Therefore, timely weed control is necessary to prevent seed production.
Keywords
Burial Depth; Cynanchum Acutum Subsp. Sibiricum; Osmotic Potential; pH; Salt; Storage; Temperature
1. Introduction
Swallowwort (Cynanchum acutum subsp. sibiricum), a member of the Apocynaceae family, is a perennial weed species with significant medicinal potential (Luo et al., 2023; Yildiz et al., 2017). This species is prevalent in Asia, commonly found in diverse habitats such as cultivated fields, orchards, fence lines, and roadsides (Li et al., 1995; Figure 1). Over recent decades, swallowwort has emerged as a challenging weed in cotton (Gossypium hirsutum L.) fields and orchards, particularly in northwest China (Sun et al., 2020). The spread of the swallowwort is attributed to its unique twining behavior, prolific seed production, vegetative propagation capabilities, and notable resistance to drought and salt stress (El-Katony et al., 2018; Nosratti et al., 2020).
Geographic distribution of swallowwort (Cynanchum acutum subsp. sibiricum) obtained from the online Global Biodiversity Information Facility (GBIF) database and the location of the study area (triangle)
As a summer perennial weed, swallowwort propagates through both seed and rhizome, demonstrating its epidemic spread and strong reproductive potential (Golzardi et al., 2014). Generally, seeds play a vital role in weed distribution, population establishment, and persistence within agroecosystems (Baskin, Baskin, 2014; Egley, Chandler, 1978). As seeds of some members of the Apocynaceae family, such as Araujia sericifera, Cionura erecta, C. acutum subsp. acutum, Gomphocarpus fruticosus, Periploca gracilis, and P. graeca (Sarikaya, Güven, 2024), swallowwort is characterized by a pair of dry follicles (sometimes reduced to one) and seeds are equipped with a silky pappus (coma). This adaptation allows swallowwort seeds to be easily dispersed by wind and water, leading to their rapid spread across the landscape through mechanisms such as surface runoff, irrigation canals, and rivers.
Understanding seed germination and emergence in response to environmental conditions is crucial for devising effective weed management strategies (Dong et al., 2020; Xiong et al., 2018). Despite the increasing problem of swallowwort globally (Meighani et al., 2021), our understanding of its seed biology and germination ecology remains limited. Comparative studies with other Cynanchum (Apocynaceae) genus species can provide valuable insights. For instance, seeds of C. acutum L. can germinate across a broad spectrum of water potentials and salinity levels, with osmotic potential up to -0.6 Mpa and salinity stress up to 300 mM having minimal impact on seed germination (Golzardi et al., 2012). Optimum germination temperature for C. acutum L. seed was 25 °C and germination did not occur at ≤ 15 °C. Maximum emergence occurred when the seeds were planted at 1.5 cm depth and seedlings did not emerge when planted at 6.5 cm depth (Pahlevani et al., 2008). In the case of C. rossicum [syn. Vincetoxicum rossicum (Kleopow) Barbar.], cold storage (4 °C) for 18 weeks reduced the germination of seeds harvested in August by 50-60%, but did not affect seeds harvested in November (DiTommaso et al., 2005).
Swallowwort is one of the most dominant pioneer species in Northwest China, particularly in Xinjiang province, which is known for being one of the world's most characteristic cold deserts, characterized by temperature-dominated drought (Yao et al., 2018; Yang et al., 2024) and soil salinization (Chen et al., 2007). Therefore, we inferred that swallowwort should possess strong adaptive mechanisms to survive in such harsh environments. The primary objectives of this study were to evaluate swallowwort seed characteristics (viability and water absorption) and the influence of various factors such as submersion, temperature, osmotic and salinity stresses, pH, and burial depth on seed germination and seedling emergence. The insights gained from this research could help to understand its potential spread and to develop effective management strategies for its control.
2. Materials and Methods
2.1 Seed collection and seed characteristics
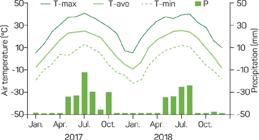
Mature follicles of swallowwort were randomly harvested in November 2017 from a natural population located along the edges of the cotton fields belonging to Tarim University (40.55° N, 81.30° E, 1,100 m a.s.l.; Figure 1), Xinjiang Province, China. Local climate data during 2017-2018 was obtained from the meteorological station at the University (Figure 2). The collection site had an extreme dry climate, with a mean annual temperature of 11.2 °C, annual rainfall of 50.9 mm, and annual sunshine hours of 2,900 h. Immediately after collection, the length and stem diameter of 130 randomly selected lanceolate follicles were measured with calipers to the nearest 0.1 mm. Seeds were extracted from the follicles, and the viable (plump) and nonviable seeds (lacking an embryo) per follicle was counted. The length and stem diameter of oblong-ovate seed and the length of white pappus (coma) were measured from 150 randomly selected plump seeds. Thousand seed weight of freshly harvested seed was estimated from four random samples of 100 plump seeds. The freshly harvested plump seeds were subject to a range of treatments outlined below. Moreover, some seeds were stored at room temperature (25 ± 1 °C) in paper bags for six months until June 2018 and were also subject to a range of treatments outlined below.
Maximum (T-max), minimum (T-min), average (T-ave) air temperatures (°C) and precipitation (P, mm) of the study area during 2017-2018
2.2 Seed viability
The viability of freshly harvested plump seeds was evaluated using a tetrazolium stain. Four samples of 100 seeds each were placed in petri dishes and immersed in a 0.1% TTC (2,3,5-triphenyl tetrazolium chloride) solution at 20 °C for 24 h in the dark (Baskin, Baskin, 2014). Prior to staining, the seed coat of each seed was scarified with a knife. Only seeds stained with a uniformly dark red embryo were classified as viable.
2.3 Water uptake during seed imbibition
To evaluate the water absorption capacity, four replicates of 50 seeds each were weighed to the nearest 0.0001 g. These were then placed in 9-cm-diam Petri dishes lined with a piece of filter paper and moistened with 20 ml distilled water at room temperature. After 1, 2, 4, 6, 8, 10, 12 and 24 hours of imbibition, seeds were immediately surface-dried with filter paper and then reweighed. This process was terminated after 48 hours of imbibition, at which time the imbibed seeds began to germinate. The percentage of water uptake was calculated as the amount of water adsorbed by the seeds relative to the initial seed weight.
where Wi and Wd are the mass of imbibed and dry seeds, respectively.
2.4 General seed germination protocol
Four replicates of 25 seeds were uniformly arranged in 9-cm-diam petri dishes on two layers of Whatman No. 1 filter paper, moistened with 4 ml of distilled water or test solutions. All dishes were sealed with Parafilm to minimize water loss and then incubated at a constant temperature of 30 °C, unless otherwise specified. The photoperiod was set at 14 hours to coincide with the typical day length between July and August, and the photosynthetic photon-flux density was maintained at 150 μmol m-2 s-1. The number of germinated seeds were counted daily for 14 days after sowing (DAS). Seeds with a radicle length of ≥ 2 mm were considered germinated. The germination percentage was calculated based on the number of germinated seeds.
2.5 Effect of submersion on seed germination
Freshly harvested seeds were submerged in 9-cm-diam petri dishes with distilled water (20 ml) in the dark for 1 to 7 days at 30/20 °C. After the submersion treatments, the number of in situ germinated seeds in water was counted. The ungerminated seeds were then subject to the seed germination test described above.
2.6 Effect of temperature on seed germination
Freshly harvested seeds (FS) and seeds stored for six months (SS) at room temperatures (20 ± 5 °C) were incubated at eight constant temperatures (10, 15, 20, 25, 30, 35, 40 and 45 °C) and eight fluctuating day/night temperatures (15/5, 20/10, 25/15, 30/20, 35/25, 40/30, 45/35 and 50/40 °C). The temperature difference between minimum and maximum in the region from March to November in recent years was approximately 10 °C (Xiong et al., 2018).
2.7 Effect of pH on seed germination
Germination was tested using FS and SS under pHs range 5, 6, 7, 8, 9, and 10, using buffered solutions prepared as the method described by Chauhan and Johnson (2008a). Unbuffered deionized water (pH 6.8) was used as a control.
2.8 Effect of osmotic stress on seed germination
To examine the effect of moisture stress on seed germination, FS and SS were placed in aqueous solutions with water potentials of 0, -0.1, -0.2, -0.4, -0.6, -0.8, and -1.0 MPa, prepared by dissolving appropriate amounts of polyethylene glycol (PEG) 8,000 in deionized water (Michel, 1983).
2.9 Effect of saline stress on seed germination
The influence of saline stress on swallowwort germination was determined by incubating FS and SS in Petri dishes containing sodium chloride (NaCl) solutions of 0, 12.5, 25, 50, 100, 150, 200, 250, and 300 mM.
2.10 Effect of burial depth on seedling emergence
The effect of seed burial depth on seedling emergence was investigated using previously published methodologies (Zhou et al., 2005). The trial was conducted in a greenhouse at 35/25 °C day/night temperature and with a 14-hour photoperiod. Twenty SS were buried at seven different depths (0 cm or surface, 1, 2, 3, 4, 6 and 8 cm) in plastic pots (15-cm-diam by 15-cm-height), filled with a 0.5:1:2 mixture of water, sandy loam and potting soil (N-P2O5-K2O ≥ 2%, organic matter ≥ 40%, pH = 6.5+0.5; Huaian Hongyang Agricultural Science and Technology Development Co., Ltd., Jiangsu Province, China). Pots were manually subirrigated as needed to maintain adequate soil moisture. Seedling emergence was defined as when both coleoptiles were visible at the soil surface. Seedlings were counted at 30 DAS.
2.11 Statistical analyses
All experiments employed a randomized complete-block design with four replications. Each experiment was conducted twice. Data from repeated experiments were subjected to ANOVA and there was no significant time-by-treatment interaction; therefore, data from two experiments were pooled for further analysis. Neither arcsine square nor log transformation of the data improved the homogeneity of variance; hence, ANOVA and regression analysis were performed on the non-transformed percentage of germination. Fisher's protected LSD test was used to identify significant differences among treatment means.
Furthermore, regression analysis was utilized to assess the impact of osmotic and salinity stresses on germination percentage. Data were fitted to a functional three-parameter logistic model (Asgarpour et al., 2015). The model applied was as follows:
where G is the total germination (%) at different concentrations of osmotic or salt potential x, Gmax is the maximum germination (%), x50 is the concentration required for 50% inhibition of the maximum germination, and Grate indicates the slope.
A three-parameter sigmoid model (Chauhan, Johnson, 2008a) was employed to describe the effects of time (days after sowing) or burial depth on germination (%). The model fitted was as followed:
where E is the total germination (%) at time or burial depth x, Emax is the maximum germination (%), x50 is the time or depth to reach 50% of maximum germination, and Erate indicates the slope.
Coefficients of determination (R2) were calculated for nonlinear regressions and used to determine the goodness of fit of nonlinear models. All probabilities were two-tailed, and the significance level was set at P = 0.05. Analysis was conducted using the statistical software SPSS 22.0.
3. Results and Discussion
3.1 Seed production
Swallowwort, like most species in the Apocynaceae family, produces comose seeds borne in lanceolate follicles (Table 1). The first follicles mature and open in October in Xinjiang province in northwest China and the plants continue to release seeds through November. Each follicle contained an average number of plump seeds 91.8 (N = 130, SE = 2.23, range 21-139) and nonviable seeds (lacking an embryo) 8.8 (SE = 0.97, range 0-62). Each seed had a silky, white, 18-50 mm long pappus (coma) at one end (Table 1), which played an important role in the dispersal of the seeds (Vijverberg et al., 2021). The thousand seeds weight of freshly harvested plump seed was 3.56 g (n = 4, SE = 0.16, range 3.19-3.91).
3.2 Seed viability
In the tetrazolium tests, 85.5% of freshly collected plump seeds were stained a dark red color and 5.2% were lightly stained red indicating high viability of freshly harvested swallowwort seeds and a maximum potential germination percentage of 90.7%.
3.3 Water uptake during seed imbibition
The relationship between seed water uptake and imbibition time (hours) was fitted with a logarithmic regression model (R2 = 0.99, P < 0.01; Figure 3). The rate of imbibition increased rapidly during the first 12 hours, with the seed mass increasing by more than 82.5%. The mass then rose steadily, increasing to 91.7% at 24 hours after imbibition. The rapid water absorption indicates the absence of a water-impermeable seed coat of swallowwort seeds. Seed imbibition dynamics have been used to determine species-specific traits or the degree of seed hardness (Abudureheman et al., 2014).
Seed mass of swallowwort (Cynanchum acutum subsp. sibiricum) during imbibition at room temperature. Vertical bars represent standard errors of the means
3.4 Effect of submersion on seed germination
When swallowwort seeds were soaked in water for more than 2 days, a portion of the seeds (6.9%) began to germinate in situ during the submersion. As the submersion duration extended, the in situ germination rate of the seeds increased gradually, from 6.9% at 2 days after submersion to 77.4% at 7 days after submersion. Most of the remaining seeds that did not germinate in situ did germinate when tested using the general germination protocol. The total germination rate, including during and post-submersion, ranged from 87.0% to 92.9%, with no difference among submersion times (P = 0.60; Figure 4). This result might indicate that O2 is not critically required for the initial germination of swallowwort seeds. For many weed species, such as hairy beggarticks (Bidens pilosa;Reddy, Singh, 1992), spotted spurge (Chamaesyce maculate;Asgarpour et al., 2015), seeds could be transferred and spread by water and submersion treatment consistently reduced seed germination compared to non-submersed conditions. In contrast, swallowwort seeds were able to germinate in water even after 2 days, with seed germination remaining unaffected by submersion. This resilience to submersion may indicate an adaptive trait of swallowwort in response to the arid climates of Northwest China. Once the seeds come into contact with water, they will germinate and establish themselves as quickly as possible.
Effect of submersion on seed germination of swallowwort (Cynanchum acutum subsp. sibiricum). Vertical bars represent standard errors of the means
3.5 Effect of temperature on seed germination
The germination of seeds was significantly influenced by both constant and alternating temperature conditions. Under constant temperatures, FS germinated across a broad temperature range, 15 to 40 °C. Elevating the temperature from 15 to 35 °C enhanced the germination of FS. Optimal seed germination was observed at 30 to 35 °C, with a germination percentage reaching 95.7 to 97.9%. No seed germination was observed at 10 or 45 °C. The seeds stored for six months at room temperature had a broader range of optimal temperatures from 15 to 35 °C, compared to the FS (30 to 35 °C). The germination percentage of the SS increased by 64.2%, 26.0%, and 17.1% at 15 °C, 20 °C, and 25 °C, respectively, compared to the FS (Figure 5A). Dry after-ripening at room temperature for six months increased seed germination of Rhodiola crenulate (Peng et al., 2023). Seeds of Silybum marianum germinated over a wider range of temperatures after two months of dry storage (Monemizadeh et al., 2021). This is a characteristic of seeds with conditional non-deep physiological dormancy (Baskin, Baskin, 2004).
Effect of constant and alternating temperature on germination of freshly harvested seeds [FS) and seeds stored for six months (SS) of swallowwort (Cynanchum acutum subsp. sibiricum). Vertical bars represent standard errors of the means. Bars with the same letters are not significantly different (p = 0.05) among different temperatures under constant or alternating temperature. Asterisk (*) indicates that the germination of freshly harvested seeds was significantly different from that of seeds stored for six months under the same temperature conditions temperature fluctuations may accelerate germination in certain flowering plants, and the amplitude of these fluctuations can influence the effectiveness of this stimulus (Thompson et al., 1977). Previous studies have also demonstrated that fluctuating day-night temperatures, which mimic natural environmental temperature changes, are more conducive to weed germination than constant temperatures (Wu et al., 2015; Xiong et al., 2018). Compared to FS, seeds stored at room temperature for six months had higher germination at extreme temperature regimes (15/5 °C) and 45/35 °C), with germination reaching 60% at 15/5 °C. In contrast, FS did not germinate at 15/5 °C.
Under alternating temperature conditions, freshly harvested swallowwort seeds exhibited germination from 20/10 °C to 45/35 °C (Figure 5B). Seed germination exceeded 94.0% at 20/10 °C, 25/15 °C, and 30/20 °C, which was greater than at correspondingly constant temperatures 15 °C, 20 °C, and 25 °C. It is well-documented that diurnal
In northwest China, swallowwort seeds mature around October and November and subsequently endure a cold winter period. After approximately five months of overwintering, the environmental temperature becomes conducive for seed germination and plant growth in the spring. It is during this period that the seeds, under favorable environmental conditions, can germinate and potentially cause problems in agricultural or horticultural systems. This study revealed that, under both constant and alternating temperature conditions, seeds stored at room temperature for six months demonstrated greater temperature adaptability compared to FS. Further research is required to determine the response of seeds to temperature after overwintering under natural conditions.
3.6 Effect of osmotic stress on seed germination
Seed germination was significantly influenced by varying osmotic potentials (P < 0.05), with a decrease in osmotic potentials leading to a reduction in seed germination (Figure 6). Germination of FS decreased from 94.7% to 2.0% as water potentials changed from 0 to -0.8 MPa. Germination was completely inhibited at an osmotic potential of -1.0 MPa, with -0.40 MPa being the osmotic potential that caused 50% inhibition of maximum germination. In contrast, SS demonstrated higher tolerance to water stress than the FS. Germination of SS could reach 84.1% at an osmotic potential of -0.8 MPa, with only a 10.9% decrease in seed germination compared to the water control. At an osmotic potential of -1.0 MPa, 56.6% germination was observed. An osmotic potential of -1.06 MPa was required to inhibit 50% of maximum germination, a value nearly close to that reported for foxtail sophora (Sophora alopecuroides; -1.0 MPa), a species that thrives under arid and semi-arid climatic conditions (Nosratti et al., 2018). Fresh seeds are more sensitive to water stress compared to stored seeds, which is consistent with the results of research on Vicia angustifolia (Zhang et al., 2014). The tolerance of a particular weed species to water stress can enhance its growth potential under dry climatic conditions (Gutterman, Gendler, 2005). Our data suggest that swallowwort exhibits high tolerance to water stress during germination and can withstand the dry soil conditions prevalent in northwest China.
Effect of water potential on seed germination of swallowwort (Cynanchum acutum subsp. sibiricum) seeds. Seeds placed in an incubator at 30 °C with a 14-h photoperiod for 14 days. Vertical bars represent standard errors of the means. FS, freshly harvested seeds; SS, seeds stored for six months
3.7 Effect of salinity stress on seed germination
Salinity reduced the germination of both FS and SS. However, the extent of this impact varied based on the treatment and salinity level. A three-parameter sigmoid model best described the relationship between seed germination and salt concentration (Figure 7). Germination of FS exceeded 67.0% at 200 mM and declined to 23.1% at 250 mM. At a salinity level of 400 mM, germination was completely inhibited. The salt concentration causing 50% inhibition of maximum germination was 226.0 mM. Seed germination of SS was higher than that of FS at all salinity levels. The salt concentration causing 50% inhibition of maximum germination was 333.9 mM. A 9% germination was achieved at the salinity level of 400 mM.
The relationship between sodium chloride (NaCl) concentration and seed germination (mean ± SE) of swallowwort (Cynanchum acutum subsp. sibiricum). Seeds placed in an incubator at 30°C with a 14-h photoperiod for 14 days. FS, freshly harvested seeds; SS, seeds stored for six months
The results demonstrated that swallowwort seeds stored for six months exhibited greater salt tolerance compared to FS at the germination stage. This phenomenon may be related to after-ripening and the loss of dormancy. In dry conditions, the water content of swallowwort seeds decreases after being stored for six months. This decrease increases the permeability of the seed coat, facilitating the infiltration of oxygen into the seeds and the exchange of internal and external gases. This process enhances respiration and energy metabolism, ultimately improving seed vitality and stress resistance (Bair et al., 2006). These seeds could germinate at a wide range of soil salinity levels, suggesting that swallowwort is fairly tolerant of salinity. Similar to swallowwort, Japanese brome (Bromus japonicus,Li et al. 2015) and giant sensitive plant (Mimosa invisa,Chauhan, Johnson, 2008b) germinated at high salt concentrations. Soil salinization is relatively severe in northwest China, especially in Xinjiang province (Yu et al., 2022), where swallowwort is a serious weed species and is widely distributed. Given its high salt tolerance, swallowwort has the potential to spread into new areas, necessitating preventive measures to control this weed.
3.8 Effect of pH on seed germination
The germination of both FS and SS was not impacted by the tested pH levels. Germination exceeded 92.9% across a broad pH range from 5 to 10 (Figure 8). This is consistent with other species capable of germinating under a wide range of pH levels, such as African mustard (Brassica tournefortii,Chauhan et al., 2006a), turnipweed (Rapistrum rugosum,Chauhan et al., 2006b), and buffalobur (Solanum rostratum,Wei et al., 2009). In northwest China, the typical soil pH range is around 8 (Liu et al., 2010). Therefore, soil pH will likely not restrict the germination process of swallowwort. This trait could potentially facilitate the colonization of various habitats by this weed species in the region.
Effect of buffered pH solution on germination of swallowwort (Cynanchum acutum subsp. sibiricum) seed incubated at 30 °C and 14 h photoperiod for 14d. FS, freshly harvested seeds; SS, seeds stored for six months
3.9 Effect of burial depth on seed emergence
The emergence of swallowwort was found to be significantly influenced by the depth of burial (P < 0.001; Figure 9). The highest seedling emergence was 71.0% when seeds were placed directly on the soil surface. Seedling emergence declined as the seed depth increased from 0 to 6 cm, with an emergence rate of 34.0% at the burial depth of 6 cm. It then dropped sharply to 2.0% at the burial depth of 8 cm. No emergence was recorded at the burial depth of 10 cm. The fitted model estimated the depth required for 50% inhibition of maximum seedling emergence to be 2.93 cm. The study found an inverse relationship between swallowwort seedling emergence and the burial depth. This pattern of emergence in relation to soil burial depth aligns well with previous studies (Rao et al., 2008, Schutte et al., 2014, Zhao et al., 2018), who have reported a decrease in seedling emergence with increasing sowing depths, and the highest emergence when seeds were sown on or near the soil surface.
Effect of burial depth on seedling emergence of swallowwort (Cynanchum acutum subsp. sibiricum). Vertical bars represent standard errors of the means
The greatest rate of seedling emergence was observed when seeds were placed directly on the soil surface. This result suggests that swallowwort may thrive in no-till and minimum-till farming systems, as these methods tend to leave a majority of weed seeds on the soil surface post-harvest. Moreover, the findings imply that if swallowwort seeds are buried deeper than 8 cm, the weed's establishment could be more challenging due to reduced emergence. Therefore, a potential management strategy for cultivating crops in fields infested with swallowwort could involve a tillage operation that buries the seeds to a minimum of 10 cm.
In summary, this study indicates that swallowwort is capable of germinating under a wide range of environmental conditions. The pH level, ranging broadly from 5 to 10, did not affect germination. Despite a decrease in germination under water stress and high salt concentration, germination still occurred across a wide spectrum of water potentials and salt concentrations. This indicates that neither water stress nor salt concentration are limiting factors for seed germination in agricultural systems. Swallowwort germination commenced at a constant temperature of 15 °C and an alternating temperature of 15/5 °C. Moreover, SS had higher tolerance to extreme environmental conditions, such as higher temperature, water potential and salt stress, resulting in better germination than the FS. Therefore, it is crucial to implement control measures against this weed species in autumn or the following spring when temperatures are conducive to germination. Manual or mechanical mowing before seed maturation in autumn is recommended (Meighani et al., 2021), with the best approach being to dig up the plants to remove roots and rhizomes. Once the follicles have opened and seed dispersal has begun, it is better to collect and incinerate the seeds. Herbicides, such as glyphosate, glufosinate (Sun et al., 2020), and triclopyr (Meighani et al., 2021), can effectively control swallowwort at the seedling stage and/or on the vines post-flowering. Seeds distributed on the soil surface are most likely to germinate, with emergence decreasing as burial depth increases. Thus, tillage operations could be an effective method of managing swallowwort.
This study has shed light on some of the environmental factors influencing seed germination and emergence, thereby contributing to our understanding of swallowwort's widespread and problematic potential in northwest China. This knowledge will aid in the development of improved control strategies in this region. However, as a perennial weed species, swallowwort is capable for both sexual reproduction by seed and vegetative reproduction through its extensive root system (Golzardi et al., 2014). A more detailed analysis of the re-growth characteristics of root will enhance our understanding of weed reproduction and contribute to the refinement of management strategies.
Acknowledgements
The authors wish to acknowledge Ying Wang and Jinqiu Sun for his assistance in the laboratory. Financial support was provided by the National Natural Science Foundation of China (Grant No. 31660525). The authors declare no conflict of interest.
References
-
Abudureheman B, Liu H, Zhang D, Guan K. Identification of physical dormancy and dormancy release patterns in several species (Fabaceae) of the cold desert, north-west China. Seed Sci Res. 2014;24(2):133-45. Available from: https://doi.org/10.1017/S0960258514000063
» https://doi.org/10.1017/S0960258514000063 -
Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10. Available from: https://doi.org/10.1614/WS-D-14-00162.1
» https://doi.org/10.1614/WS-D-14-00162.1 -
Bair NB, Meyer SE, Allen PS. A hydrothermal after-ripening time model for seed dormancy loss in Bromus tectorum L. Seed Sci Res. 2006;16(1):17-28. Available from: https://doi.org/10.1079/SSR2005237
» https://doi.org/10.1079/SSR2005237 - Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. 2nd ed. San Diego: Academic Press; 2014.
-
Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Sci Res. 2004;14(1):1-16. Available from: https://doi.org/10.1079/SSR2003150
» https://doi.org/10.1079/SSR2003150 -
Chauhan BS, Gill G, Preston C. African mustard (Brassica tournefortii) germination in southern Australia. Weed Sci. 2006a;54(5):891-97. Available from: https://doi.org/10.1614/WS-06-053R.1
» https://doi.org/10.1614/WS-06-053R.1 -
Chauhan BS, Gill G, Preston C. Factors affecting turnipweed (Rapistrum rugosum) seed germination in southern Australia. Weed Sci. 2006b;54(6):1032-36. Available from: https://doi.org/10.1614/WS-06-060R1.1
» https://doi.org/10.1614/WS-06-060R1.1 -
Chauhan BS, Johnson DE. Germination ecology of goosegrass (Eleusine indica): an important grass weed of rainfed rice. Weed Sci. 2008a;56(5):699-706. Available from: https://doi.org/10.1614/WS-08-048.1
» https://doi.org/10.1614/WS-08-048.1 -
Chauhan BS, Johnson DE. Seed germination and seedling emergence of giant sensitiveplant (Mimosa invisa) Weed Sci. 2008b;56(2):244-48. Available from: https://doi.org/10.1614/WS-07-120.1
» https://doi.org/10.1614/WS-07-120.1 - Chen X, Yang J, Liu C, Hu S. Soil salinization under integrated agriculture and its countermeasures in Xinjiang. Soils. 2007;39:347-53.
-
DiTommaso A, Brainard DC, Webster BR. Seed characteristics of the invasive alien vine Vincetoxicum rossicum are affected by site, harvest date, and storage duration. Can J Bot. 2005;83(1):102-10. Available from: https://doi.org/10.1139/b04-154
» https://doi.org/10.1139/b04-154 -
Dong H, Ma Y, Wu H, Jiang W, Ma X. Germination of Solanum nigrum L. (Black nightshade) in response to different abiotic factors. Planta Daninha. 2020;38:1-12. Available from: https://doi.org/10.1590/S0100-83582020380100049
» https://doi.org/10.1590/S0100-83582020380100049 -
Egley GH, Chandler JM. Germination and viability of weed seeds after 2.5 years in a 50-year buried seed study. Weed Sci. 1978;26(3):230-9. Available from: https://doi.org/10.1017/S004317450004978X
» https://doi.org/10.1017/S004317450004978X -
El-Katony TM, Khedr AHAF, Mergeb SO. Drought stress affects gas exchange and uptake and partitioning of minerals in swallowwort (Cynonchum ocutum L.). Rend Fis Acc Lincei. 2018;29:23-34. Available from: https://doi.org/10.1007/s12210-017-0654-7
» https://doi.org/10.1007/s12210-017-0654-7 - Golzardi F, Vazan S, Moosavinia H, Tohidloo G. Effects of salt and drought stresses on germination and seedling growth of swallow wort (Cynonchum ocutum L.). Res J Appl Sci Eng Technol. 2012;4(21):4524-9.
- Golzardi F, Vaziritabar Y, Vaziritabar Y, Tafti SF, Asilan KS, Sayadi MHJ et al. Investigation of drying effect on developing growth of two Cynonchum ocutum L. populations. Inter J Adv Life Sci. 2014;7(4):680-5.
-
Gutterman Y, Gendler T. Annual rhythm of germination of seeds of Mesembryanthemum nodiflorum 32 years after collection. Seed Sci Res. 2005;15(3):249-53. Available from: https://doi.org/10.1079/SSR2005215
» https://doi.org/10.1079/SSR2005215 - Li PT, Michael GG, Stevens WD. Asclepiadaceae. In: Wu Z, Raven P, editors. Flora of China. Beijing: Science Press; 1995. p. 311-13
-
Li Q, Tan J, Li W, Yuan G, Du L, Ma S, Wang J. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus joponicus) Weed Sci. 2015;63(3):641-6. Available from: https://doi.org/10.1614/WS-D-14-00131.1
» https://doi.org/10.1614/WS-D-14-00131.1 -
Liu E, Yan C, Mei X, He W, Bing SH, Ding L et al. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma. 2010;158(3-4):173-80. Available from: https://doi.org/10.1016/j.geoderma.2010.04.029
» https://doi.org/10.1016/j.geoderma.2010.04.029 -
Luo D, Wang X, Lei Y, Lu C, Lu Y, Chen D et al. Cynasibirolide A, one new humulanolide sesquiterpene from Cynonchum ocutum subsp. sibiricum Chem Biodivers. 2023;20(3). Available from: https://doi.org/10.1002/cbdv.202200860
» https://doi.org/10.1002/cbdv.202200860 -
Meighani F, Karaminejad MR, Farrokhi Z. Invasive weed swallow-wort (Cynonchum ocutum L.) response to chemical and mechanical practices. Weed Biol Manag. 2021;21(2):124-32. Available from: https://doi.org/10.1111/wbm.12231
» https://doi.org/10.1111/wbm.12231 -
Michel BE. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983;72(1):66-70. Available from: https://doi.org/10.1104/pp.72.1.66
» https://doi.org/10.1104/pp.72.1.66 -
Monemizadeh Z, Ghaderi-Far F, Sadeghipour HR, Siahmarguee A, Soltani E, Torabi B et al. Variation in seed dormancy and germination among populations of Silybum morionum (Asteraceae). Plant Species Biol. 2021;36(3):412-24. Available from: https://doi.org/10.1111/1442-1984.12326
» https://doi.org/10.1111/1442-1984.12326 -
Nosratti I, Amiri S, Bagheri A, Chauhan BS. Environmental factors affecting seed germination and seedling emergence of foxtail sophora (Sophoro olopecuroides) Weed Sci. 2018;66(1):71-7. Available from: https://doi.org/10.1017/wsc.2017.35
» https://doi.org/10.1017/wsc.2017.35 -
Nosratti I, Sabeti P, Chaghamirzaee G, Heidari H. Weed problems, challenges, and opportunities in Iran. Crop Prot. 2020;134. Available from: https://doi.org/10.1016/j.cropro.2017.10.007
» https://doi.org/10.1016/j.cropro.2017.10.007 -
Pahlevani AH, Rashed MH, Ghorbani R. Effects of environmental factors on germination and emergence of swallowwort. Weed Technol. 2008;22(2):303-8. Available from: https://doi.org/10.1614/WT-07-0551
» https://doi.org/10.1614/WT-07-0551 -
Peng DL, Geng BY, Qin YB, Yang LE, Baskin JM, Baskin CC. Non-deep physiological dormancy in seeds of two endangered medicinal alpine species of Rhodiolo from the Hengduan Mountains of southwest China. Seed Sci Technol. 2023;51(3):297-303. Available from: https://doi.org/10.15258/sst.2023.51.3.02
» https://doi.org/10.15258/sst.2023.51.3.02 -
Rao N, Dong L, Li J, Zhang H. Influence of environmental factors on seed germination and seedling emergence of American sloughgrass (Beckmonnio syzigochne) Weed Sci. 2008;56(4):529-33. Available from: https://doi.org/10.1614/WS-07-158.1
» https://doi.org/10.1614/WS-07-158.1 -
Reddy KN, Singh M. Germination and emergence of hairy beggarticks (Bidens piloso) Weed Sci. 1992;40(2):195-9. Available from: https://doi.org/10.1017/S0043174500057210
» https://doi.org/10.1017/S0043174500057210 -
Sarikaya E, Güven S. Contributions to the taxonomy of Periplocoideae and Asclepiadoideae (Apocynaceae) in Türkiye based on fruit and seed morphology. Plant Biosys Int J Deal Aspects Plant Biol. 2024;158(5):963-77. Available from: https://doi.org/10.1080/11263504.2024.2383449
» https://doi.org/10.1080/11263504.2024.2383449 -
Schutte BJ, Tomasek BJ, Davis AS, Andersson L, Benoit DL, Cirujeda A et al. An investigation to enhance understanding of the stimulation of weed seedling emergence by soil disturbance. Weed Res. 2014;54(1):1-12. Available from: https://doi.org/10.1111/wre.12054
» https://doi.org/10.1111/wre.12054 - Sun J, Ma Y, Ren X, Hu H, Jiang W, Ma Y et al. [Control efficacy of three herbicides on Cynonchum sibiricum at different growth stages]. China Cott. 2020;47(9):5-7. Chinese.
-
Thompson K, Grime JP, Mason G. Seed germination in response to diurnal fluctuations of temperature. Nature. 1977;267(5607):147-9. Available from: https://doi.org/10.1038/267147a0
» https://doi.org/10.1038/267147a0 -
Vijverberg K, Welten M, Kraaij M, van Heuven BJ, Smets E, Gravendeel B. Sepal identity of the pappus and floral organ development in the common dandelion (Taraxacum officinale; Asteraceae). Plants. 2021;10(8):1-26. Available from: https://doi.org/10.3390/plants10081682
» https://doi.org/10.3390/plants10081682 -
Wei S, Zhang C, Li X, Cui H, Huang H, Sui B et al. Factors affecting buffalobur (Solonum rostrotum) seed germination and seedling emergence. Weed Sci. 2009;57(5):521-5. Available from: https://doi.org/10.1614/WE-09-054.1
» https://doi.org/10.1614/WE-09-054.1 -
Wu X, Li J, Xu H, Dong L. Factors affecting seed germination and seedling emergence of asia minor bluegrass (Polypogon fugox) Weed Sci. 2015;63(2):440-7. Available from: https://doi.org/10.1614/WS-D-14-00093.1
» https://doi.org/10.1614/WS-D-14-00093.1 -
Xiong R, Ma Y, Wu H, Jiang W, Ma X. Effects of environmental factors on seed germination and emergence of velvetleaf (Abutilon theophrosti) Planta Daninha. 2018;36:1-13. Available from: https://doi.org/10.1590/S0100-83582018360100122
» https://doi.org/10.1590/S0100-83582018360100122 -
Yang JH, Li YQ, Zhou L, Zhang ZQ, Zhou HK, Wu JJ. Effects of temperature and precipitation on drought trends in Xinjiang, China. J Arid Land. 2024;16:1098-117. Available from: https://doi.org/10.1007/s40333-024-0105-0
» https://doi.org/10.1007/s40333-024-0105-0 -
Yao J, Zhao Y, Chen Y, Yu X, Zhang R. Multi-scale assessments of droughts: a case study in Xinjiang, China. Sci Total Environ. 2018;630:444-52. Available from: https://doi.org/10.1016/j.scitotenv.2018.02.200
» https://doi.org/10.1016/j.scitotenv.2018.02.200 -
Yildiz I, Sen O, Erenler R, Demirtas I, Behcet L. Bioactivity-guided isolation of flavonoids from Cynanchum acutum L. subsp. sibiricum (willd.) Rech. f. and investigation of their antiproliferative activity. Nat Prod Res. 2017;31(22):2629-33. Available from: https://doi.org/10.1080/14786419.2017.1289201
» https://doi.org/10.1080/14786419.2017.1289201 -
Yu X, Lei J, Gao X. An over review of desertification in Xinjiang, Northwest China. J Arid Land. 2022;14(11):1181-95. Available from: https://doi.org/10.1007/s40333-022-0077-x
» https://doi.org/10.1007/s40333-022-0077-x - Zhang R, Li TS, Hu XW, Wang YR. [Seed dormancy and germination characteristics of Vicia angustifolia] Acta Bot Bor Occid Sin. 2014;34(1):135-42. Chinese.
-
Zhao N, Li Q, Guo W, Zhang L, Ge L, Wang J. Effect of environmental factors on germination and emergence of shortawn foxtail (Alopecurus aequalis) Weed Sci. 2018;66(1):47-56. Available from: https://doi.org/10.1017/wsc.2017.42
» https://doi.org/10.1017/wsc.2017.42 -
Zhou J, Deckard EL, Messersmith CG. Factors affecting eastern black nightshade (Solanum ptycanthum) seed germination. Weed Sci. 2005;53(5):651-6. Available from: https://doi.org/10.1614/WS-04-168R2.1
» https://doi.org/10.1614/WS-04-168R2.1
Edited by
-
Editor in Chief:
Carol Ann Mallory-Smith
-
Associate Editor:
Michaela Kolářová
Publication Dates
-
Publication in this collection
31 Mar 2025 -
Date of issue
2025
History
-
Received
07 Nov 2024 -
Accepted
03 Feb 2025

 Seed and germination biology of swallowwort (Cynanchum acutum subsp. sibiricum)
Seed and germination biology of swallowwort (Cynanchum acutum subsp. sibiricum)