ABSTRACT
The points of susceptibility of seeds to deterioration are important factors, especially when stored in hot and humid tropical conditions. The seeds of andiroba (Carapa guianensis) are an important resource for Amazonian traditional communities, as the oil extracted from the seeds is widely used in popular medicine and the cosmetic industry. Yet andiroba seeds are subject to fungal spoilage, which affects the quality of the oil. We analyzed the morpho-histological characteristics of whole andiroba seeds by stereo and scanning electron microscopy to identify susceptibility points to fungi. The shell (tegument), a thick lignin-rich, protective wall structure, varied in the type and number of tissue layers, with polygonal and long shaped cells presenting pores (plasmodesmata). The junction of the three faces of the seeds at the top formed a relatively tight small cavity, the micropyle, rich in vascular bundles of helically and tracheid-shaped vessels. An interchange channel from the pod through the micropyle to the inner seed is formed between the shell and a thin brown skin towards the cotyledons, with its surface covered with adhered residues of dry placental tissue. The seed cotyledons (cross and longitudinal sections), presented different cell layers containing randomly distributed lipid droplets. We concluded that the highly irregular surface of the micropyle and channel at the seed top, which forms deep recesses and accumulates tissue residues, presents the righest risk for fungi conidia deposition and/or moisture absorption, which leads to spoilage.
KEYWORDS:
Amazon; anatomical structures; morphology; microscopy
RESUMO
Os pontos de suscetibilidade das sementes à deterioração são fatores importantes, especialmente quando armazenadas em condições tropicais quentes e úmidas. As sementes de andiroba (Carapa guianensis) são um recurso importante para as comunidades tradicionais amazônicas, pois o óleo extraído das sementes é amplamente utilizado na medicina popular e na indústria de cosméticos. Porém, as sementes de andiroba são suscetíveis à deterioração por fungos, o que afeta a qualidade do óleo. Nós analisamos as características morfo-histológicas de sementes inteiras de andiroba por meio de microscopia estereoscópica e eletrônica de varredura para identificar pontos de suscetibilidade a fungos. A casca (tegumento), uma estrutura protetora espessa e rica em lignina, variou no tipo e no número de camadas de tecido, com células de formato poligonal e alongado apresentando poros (plasmodesmos). A junção das três faces da semente no topo formou uma pequena cavidade relativamente fechada, o micrópilo, rico em feixes vasculares com vasos em espiral e de formato traqueidal. Um canal de intercâmbio da vagem até a semente interna é formado entre a casca e uma fina membrana marrom em direção aos cotilédones, cuja superfície é coberta por resíduos aderidos de tecido placentário seco. Os cotilédones da semente (seções transversais e longitudinais) apresentaram diferentes camadas celulares contendo gotas lipídicas distribuídas aleatoriamente. Concluímos que a superfície altamente irregular do micrópilo e o canal no topo da semente, que formam recessos profundos e acumulam resíduos de tecido, apresentam maior risco para a deposição de conídios de fungos e/ou absorção de umidade, levando à deterioração.
PALAVRAS-CHAVE:
Amazônia; estruturas anatômicas; morfologia; microscopia
INTRODUCTION
The Amazon forest is rich in plants with bioactive compounds, and their rational exploitation contributes to the sustainable development of the region (Cabral et al. 2013). Andiroba (Carapa guianensis Aubl.) (Meliaceae) is a versatile and multipurpose tree (Dias et al. 2023) with great economic value in the Amazon, ranging from the use of its wood, which has a high value in the furniture and construction industries (Firmino et al. 2019), to the use of its seeds for biodiesel production (Moura 2019) and in the cosmetic industry (Mendonça and Ferraz 2007).
The oil extracted from andiroba seeds is in high demand due to its numerous properties and applications in popular medicine and the cosmetic sector (Mendonça and Ferraz 2007; Kenfack 2011; Silva and Scussel 2020). Additionally, it is effective in pest control against insects (Klauck et al. 2015), mites and parasites (Moraes et al. 2010), as well as protozoa (Pereira et al. 2014), and fungi (Sousa et al. 2018; Silva et al. 2019), among others.
It is thus an important non-timber forest product, which is produced mainly by traditional communities, representing a sustainable way for economic development in the Amazon (Ferraz et al. 2002). Due to the great interest of the market, the oil production chain is increasingly demanded to improve the quantity and quality of forest products to meet the requirements of consumer markets (Alves 2010).
Although there is no published evidence specifically regarding fungal attacks on stored andiroba seeds or the reduction in oil quality from contaminated seeds, there are examples for other species such as cacao (Theobroma cacao L.), the stored seeds of which can be attacked by Aspergillus and Penicillium fungi leading to the deterioration of the beans and a reduction in the quality of the final product, such as chocolate and its derivatives (Kreibich et al. 2017a; 2017b). Similarly, Brazil nut (Bertholletia excelsa HBK) seeds are also susceptible to fungal contamination, especially during storage, when fungi like Aspergillus flavus Sacc. compromise the quality of the nuts and pose a health risk due to the production of aflatoxins (Manfio et al. 2012; Scussel et al. 2014a; 2014b). These examples highlight the importance of controlling fungal contamination in seeds.
Andiroba trees produce roundish fruit pods of 4-6 valves containing polygonal to irregularly-shaped seeds of varying size, measuring around 3-5 cm and weighing around 21 g (Lorenzi 2002; Tsukamoto et al. 2019; Silva and Scussel 2020). A microscopical morpho-histological analysis of the seed can be instrumental in identifying points of weakness for fungal attachment and infestation (Scussel et al. 2014a; Kreibich et al. 2017a). For andiroba, only the anatomy of adult and seedling leaves through light, scanning electron and force microscopy is known (Aragão et al. 2014; Matos et al. 2021).
Aspects of the anatomy and morphology of the andiroba seed have been reported in studies on the taxonomy of Carapa, as well as the biology and germination technology of its seeds, based on optical microscopy analysis (Fisch 1990; Ferraz et al. 2002; Carvalho 2008; Kenfack 2011). These studies have highlighted characteristics of the locule and seed size, noting the presence of a larger hilum without a delimiting protrusion and the attachment of placental tissue residues (Fisch 1990; Kenfack 2011). Ferraz et al. (2002) identified that the cotyledons form a single reserve mass due to the fusion of their parts, which complicates the perception and separation of these structures. Additionally, the embryonic axis is small and located within the cotyledonary tissue, near the micropyle (Ferraz et al. 2002).
Given the complexity and importance of the anatomical structures of the andiroba seed, more detailed microscopic studies are necessary to expand our understanding regarding resistance to pathogens, germination efficiency, and oil quality, contributing to the economic valuation and conservation of the species in the Amazon. By employing modern techniques such as scanning electron microscopy, it is possible to identify morphological characteristics that are not visible with stereoscopic and optical microscopy. Considering the lack of information on the microanatomy of andiroba seeds, this study investigated the morpho-histological characteristics of the outer and inner surfaces, as well as the tissues of the shell and cotyledons, using optical and scanning electron microscopy to identify points susceptible to fungal infection.
MATERIAL AND METHODS
Andiroba seeds (350 g) were donated by an artisanal producer in Ramal do Branco (2°46’46.5”S, 59°22’01.9”W), municipality of Rio Preto da Eva, Amazonas State, Brazil. The whole seeds were dehydrated (105 °C for 4 hours to a final moisture content of 1.9%) and then the fat was extracted from the cotyledon by adding acetone (30 min), stirred and air dried, and then prepared for analysis through stereo microscopy (SM) and scanning electron microscopy (SEM).
Stereo microscopy
For the morphological analysis through SM, the seeds were sectioned into halves in longitudinal and cross section (plans 1 and 2, respectively, in Figure 1a,b) using a fine saw. The shell (including the thin brown skin) and the cotyledon were detached and sectioned using a stainless steel scalpel. We used a stereo microscope (OPTZTS, Opticam - Tokyo, Kt., Japan) to observe the outer and inner surface, as well as the longitudinal and cross sections, of the seed shell and cotyledon surfaces under different amplifications (7.1 to 115 X).
Andiroba (Carapa guianensis) seeds showing the planes of longitudinal and transverse cross section (Plans 1 and 2, respectively) for SM and SEM analysis. The faces (A to D) and tips (junction points of three faces) (tips 1 to 4) are also shown in different perspectives. Face A is curved and forms the seed face to pod contact, whilefaces B, C, D are straight and form the face to face contacts within the pod. Stereo microscopy images at x0.67 magnification.
Scanning electron microscopy
The morpho-histologycal analysis of the main structures, tissues and cells through SEM (JEOL JSM-6390LV - Peabody, Mass., USA) followed Scussel et al. (2014a). The shell was cut into small cubes (about 3 x 3 x 2 mm in width / length / thickness, respectively), including special features such as the seed tip at the locule in the testae (tip 4 in Figure 1). The cotyledons were sliced (3 to 4 mm thick) at different points (including the area close to the junction of the two cotyledons). The cubes were fixed on stubs containing double-sided carbon tape and coated with a layer (40 nm) of gold (Au) under vacuum on a planetary basis (EM-Scd500, Leica - Leider, III., USA), then scanned and observed at different magnifications (up to 3,000 X) (Scussel et al. 2014a; Kreibish et al. 2017a). The analysis was carried out at the Electronic Microscopy Laboratory Center at Universidade Federal de Santa Catarina.
RESULTS
Seed morphology
Due to the irregular shape of the seeds, they presented multiple faces varying in size, forming angled corners at the interface of two faces and tips at the encounter of three faces (Figure 1). Face A (Figure 1) is the largest and has a curved shape as it is in direct contact with the pod wall. Faces B, C and D (Figure 1) are straight and form the contact interfaces among seeds in the pod. The micropyle is a quite tight opening formed at the top junction of faces B, C and D (tip 4, Figure 1).
The seeds had an irregular shape with multiple faces of varying sizes, forming angled corners and tips at the junctions of three faces (Figure 1). Face A was the largest, with a curved shape due to its contact with the pod wall, while Faces B, C, and D were straight and formed the contact interfaces between seeds. The micropyle was a narrow opening located at the top junction of these three faces (tip 4, Figure 1).
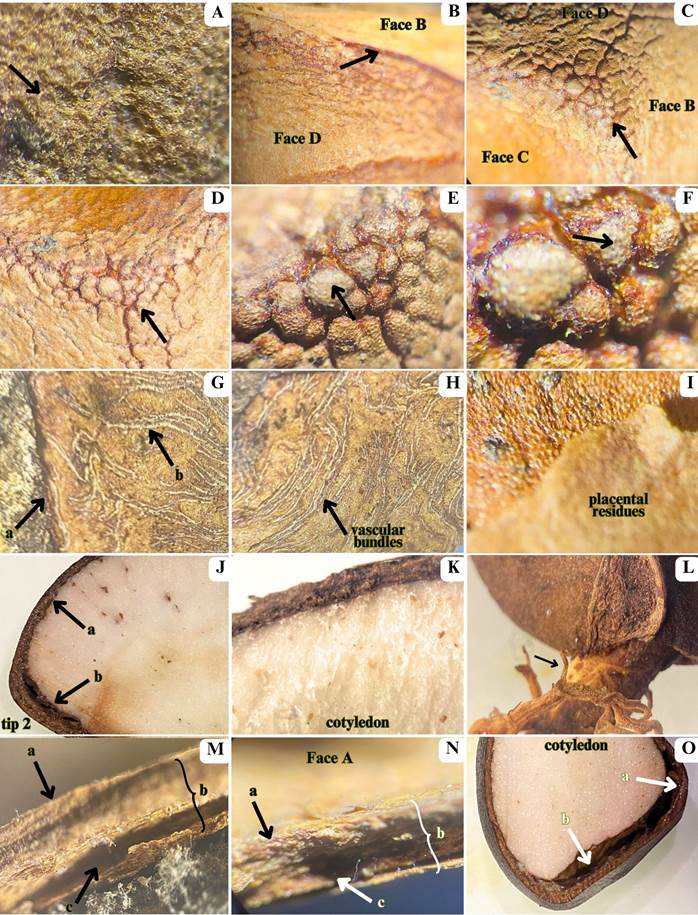
The seed shape resulted from internal pressure within the pod, with four tips at the upper junctions of the faces where the seed’s navel connected it to the pod (Figure 1). The number, type, and color of tissue layers varied across the faces, face corners, tips, and the micropyle (Figure 2). The shell surface ranged from rough to smooth and waxy (Figure 2a-f). Face A had a velvety surface with some placental residues attached (Figure 2i), while Faces B, C, and D were smoother, with consistently corrugated edges, forming irregular lobes and recesses, especially at the micropyle (tip 4, Figure 2c-d).
Stereo microscopy images of andiroba (Carapa guianensis) seeds. A - central face region showing polygonal and hexagonal-shaped cells (arrow); B-D - shell tips and tissue positioning illustrating tissue connections (arrows); E-F - presence of recesses and lobes (arrows); G-H - brown skin (arrow a) and vascular bundles (arrow b); I - outer placental surface adhered to the shell; J - external shell (arrow a) and spongy tissue layers (arrow b); K - cotyledon; L - seed germination; M-N - external surface of the shell (arrow a), shell layers (arrow b), and brown skin (arrow c); O - cotyledon connection (arrow a) and micropyle channel (arrow b). Magnification 7.1-115X.
In the cross and longitudinal sections, the shell layer was thinner in faces B, C, D and tips 1-3 than in face A and tip 4 (where the micropyle is located) (Figure 2o). All shell layers were brown, except one that was white to light creamy (Figure 2j-k,o). In longitudinal section, it was possible to visualize details of a channel starting from the micropyle at tip 4 (Figure 2o). A much more compact channel also occurs at the other tips (Figure 2j-k,o). In germinated seeds, it was possible to observe the white, cylindrical, smooth and glabrous radicle and the primary roots (Figure 2l). As expected, it was not possible to clearly detect by SM the number and details of seed tissue layers of cotyledons and embryos by SM in cross or longitudinal section as they are too close to each other. The cotyledon tissue was all-white to light creamy (Figure 2j-k,o).
Seed tissue layers and histological characteristics
Most shell tissues were constituted of thick-walled protective cells (sclerenchyma and collenchyma) (Figures 3-6). The outer surface of all seed faces and the micropyle appeared as a rather amorphous, waxy, thin tissue of polygonally shaped cells (Figure 3h), and a thick wall with no clearly visible distinction among the cells of this layer - the cuticle (Figure 3a,b). Beyond the presence of these two layers, the surface relief of the faces varied, being plain at the center, and wavy, with protrusions and recesses at the corners (Figure 3c-f). The micropyle presented a similar surface tissue. The corners feature polygonal cells of multiple tissues and long cell tissues, exhibiting placental residues adhered to the surface (Figure 3k,l). The face linkage points form several lobe-like bumps that end in a narrow cavity that is partially filled with helical tubes (xylem and phloem vascular bundles) (Figure 4a,b,e,f; Figure 5d). This cavity forms the connection between the external environment and the cotyledon through the channel filled with vascular cambium. The uneven surface of the thickned faces at the tips forms recesses and deep valleys where fungi conidia can get trapped (Figure 3e,f). The linkage surface of face A presented polygonally shaped cells (Figure 3b), face B showed cells with amorphous shape (Figure 3g), and the linkage surface of face C presented long or multiformed cells (Figure 3i). The long cells were porous and presented scars (Figure 3j). The inner surface of the shell presented cells with a more delimited, polygonal shape with a thin brown skin attached to it (Figure 2g,m-n). Vascular bundles were distributed all over the inner surface (Figure 4c,d).
Scanning electron microscopy images of andiroba (Carapa guianensis) seed shell. A-B - tissue faces featuring polygonal and hexagonal cell shapes (arrow a) alongside a smooth surface of homogeneous waxy tissue (arrow b); C - uneven, rough formations resembling lobes (arrow b) with deep valleys (arrow a) where placental residues adhere (arrow c); D - residues (arrow a) lodged between the rough shell surface and within the valleys among hexagonal cells (arrow b); E-F - locule within the testae tissue marking the micropyle entrance, characterized by rough protrusions and lobes (arrows); G - connection between shell faces and micropyle with polygonal-shaped cells (arrow a), amorphous cells (arrow b), and junction of two corner faces; H - multiple cell types near tips, highlighting polygonal cells with a thin waxy membrane (arrow); I - linkage surface tissue comprising elongated cells (arrow a) and variously shaped cells (arrow b); J - long, porous cells (arrow a), along with scars (arrow b) and pores (arrow c); K-L - multi-tissue polygonal corners (arrow a) and long cell tissues (arrow b), featuring placental residues adhered to the surface (arrow c). Magnification 150-900X.
Scanning electron microscopy images of the seed shell of andiroba (Carapa guianensis in cross section. A - inner view of the micropyle showing vascular bundles (arrow); B - vascular bundles in the micropyle formed by helical tubes (trackeid wall tickening); C - inner shell tissue showing hexagonal (arrow a) and long (arrow b) cells and thick walls; D - mass of polygonal and long cells in the inner shell tissue; E - system of vessels (arrow) with vascular bundles (helical tubes); F - channel with helical and tracheid tubes (arrow). Magnification 150-900X.
Scanning electron microscopy images of the seed shell of andiroba (Carapa guianensis) in cross section. A - tissue layers from outer to inner surfaces; B - main surfacelayers; C - tissue layer cells with globose and long shape (arrow a), and thick walls with pores and scars (arrow b); D - region with vascular bundles rich in helical tubes; E - cell tissue presenting plasmodesmata globose cells with pores and scars (arrow) and multi-layered wall (circle); F - detail of plasmodemata cell wall layers and cell content (arrow) and multi-layered wall (circle). Magnification 300- 3000X.
In cross section, the shell was composed of our main tissue layers. The primary layer was composed of long cells with a thick, porous wall (Figure 5a,b); the secondary layer presented cells with a globous to hexagonal shape (Figure 5a,b), with cells of globose and elongated shapes, with thick walls that contain pores and scars (Figure 5c). They are rich in vascular bundles (Figure 5d) and feature plasmodesmata cell walls and multi-layered walls (Figure 5f), with specialized pores (Figures 3j, 6e) for cell-to-cell communication through microscopic channels that traverse the cell wall, allowing for intercellular transport (Maule 2008). We observed cell wall scars produced by cell detaching from each other and depressions where the wall was thinned out (Figure 6a-f). At the face corners, where the layers thickened, probably to confer higher shell resistance for cotyledon protection (Scussel et al. 2014a), we observed an additional layer of a sponge-like tissue with a mix of irregularly shaped cells (mainly polygonal and long) (Figure 4c,d) and vascular bundles (helically shaped structures). At the junction of faces B, C and D (which formed the broadest corner that holds the locule in the testae) we observed the micropyle and the channel containing vascular tissue that forms the vascular bundles of the protoxylem and phloem (Figure 4a,b,e,f).
Scanning electron microscopy images of shell tissues of the seed of andiroba (Carapa guianensis) A and B - vascu1ar tissue showing porous globose cells with adhered placental residue (arrow in B); C - secondary tissue showing porous long cells; D - primary tissue showing plasmodesmata pores (arrow); E and F - thick wall layers with pores (arrow in F). Magnification 1000-4000 X.
In the cotyledon, the endosperm tissue was composed of an epidermis layer with a series of thick walls formed by bi and tri-palisade cells arranged with the long diameter at right angles to the median plane (Figure 7a,b). The first parenchymal layer contained shorter cells, more variable in width, with smaller size and somewhat thicker wall than in the second layer (Figure e,f), possibly having more of a functional activity than a storage role. The second layer consisted of larger, more globoid or polygonal, thinner-walled cells occupuing most of the cotyledon towards the seed’s center (Figure 7c,d), as well as the cortex and medullary tissues. Between the two parenchymal layers, a thin ring of meristematic tissue was present, composed of layers of small, uniformly sized, elongated cells forming the provascular tissue (Figure 7e,f), along which rudimentary vascular bundles were arranged.
Scanning electron microscopy images of the cotyledon of seeds of andiroba (Carapa guianensis L.). A and B - endosperm tissue with cavities (epidermis); C and D - endosperm and parenchymal tissue with polygonal and round cells; E and F - tissue layer with thin-walled, polygonal and globose parenchymal cells (arrow in F). Magnification 150-900X.
DISCUSSION
As observed in our study, the shell surface of andiroba seeds has a quite uneven relief, allowing humidity accumulation and absorption, favoring the adherence and development of fungal conidia. The conditions for deterioration processes may be further ehanced by cracks that occur during pods falling onto the ground and micro-cracks forming during dehydrations, as reported for Brazil nuts and cocoa beans (Scussel et al. 2014a-b; Kreibich and Scussel 2015).
The very humid (around 80% relative humidity), hot (up to 40 °C), and rainy conditions of the Amazon rainforest favor the natural proliferation of fungi (Segovia et al. 2011), creating ideal conditions for their growth. The pods are vulnerable to birds, rodents, and monkeys (Almeida et al. 2009), which not only consume the pods but can also contaminate them with microbial inocula through their feces, regurgitation, saliva, and even soil particles on their limbs. As the pod valves begin to open after falling to the ground, they expose the seeds to dust, soil materials, and moisture, further increasing the risk of microbial contamination at the time of collection (Silva and Scussel 2020). The proliferation of fungi and other microorganisms can be exacerbated by insects, especially moths of the Hypsipyla genus (Guariguata et al. 2000; Plowden 2004), which play a significant role in increasing the diversity of the contaminant microbiota (Almeida et al. 2009). This ecological interaction leads to seed predation by insects, which directly impacts seed quality, resulting in reduced germination potential (Crawley and Gillman 1989; Pinto 2007). This not only affects species regeneration but also oil production, as predation reduces the number of available seeds, impacting both the quantity and, indirectly, the quality of the oil produced (Santos and Pellicciotti 2016).
The characteristics of the outer shell of the andiroba seed described in here coincide with those reported by Kenfack (2011) regarding the structural characteristics of the locule and the size, lack of a delimiting protrusion and presence of attached residues of placental tissues at the hilum. This indicates that the presence of placental residues in the rugged surface at the tips and corners of the seed is a common occurrence that forms a high risk site for attachment and growth of microorganisms.
A seed shell formed by four main tissue layers that increase in number as they link at the face corners was also reported for the shell Brazil nut seeds (Scussel et al. 2014a,b). In our study, it was not possible to clearly discern the number and details of seed tissue layers nor the two cotyledons or embryo region as they were too close to each other, which was also reported by Ferraz et al. (2002).
We observed the presence of sclerenchyma and collenchyma, which are thick-walled protective cells within the shell tissue. The sclerenchyma provides rigid support, while the collenchyma offers flexible support properties to the shell’s layers. Additionally, there are specialized cells that create a filtering mechanism, acting as a pressure vessel to prevent over-expansion when water enters the porous cells. This structure is part of the seed coat’s protective mechanism, facilitating and regulating the exchange of gases and water between the seed and its environment (Scussel et al. 2014a; Kreibish et al. 2017a).
The presence of helical tubes (primary xylem and phloem vascular bundles) in the internal structure of the seed was also described for Brazil-nut seeds (Scussel et al. 2014a). These tubes form an input connection between the external environment and the endosperm, through a channel full of vascular bundles (cambial system), with the function of transporting and distributing nutrients and water to the seed while still attached to the tree (Scussel et al. 2014a).
The micropyle is a point of the seed where the tegument does not completely close, forming a cavity close to the hilum region (Camargo et al. 2008). During dehydration, the micropyle becomes hollower, facilitating the interference of external factors. Thereforeit’s the micropyle and its associated channel to the inner seed are the main entrance way for microorganisms and moisture in nuts (Scussel et al. 2014b). The cotyledons are fused in a single reserve mass, making it impossible to perceive and separate the two parts (Scussel et al. 2014a). The embryonic axis is very small and located within the cotyledonary tissue close to the micropyle (Ferraz et al. 2002). Although lipids are distributed throughout the cotyledons, they are present in higher concentration closer to the embrionary axis and the endosperm (Carvalho and Nakagawa 2000).
To control and/or prevent attacks of insects and the proliferation of fungi in andiroba seeds, several procedures should be applied after the pods fall from the tree. Manfio et al. (2012) have recommended to set a suspended net around the tree trunks for pod collection to prevent them from falling to the ground. This measure would prevent crack formation, reduce seed exposure to humidity and mud, and minimize ground insect attacks, reducing problems with post-collection cleaning procedures. Packaging the seeds in jute bags for transportation by boat from the collection sites could improve ventilation and/or adequate exhaustion in the boat storage room and better control humidity and the proliferation of insects and fungi (Manfio et al. 2012; Scussel et al. 2012b; Kreibich, 2017b). Further recommended preventative measures are the cleaning and drying of the seeds as soon as they arrive at the processing facility by immersing them in water for at least 24 hours to eliminate larvae by drowning and then deshelling, dry storing in seed bags and dispatching as soon as possible for oil extraction (Manfio et al. 2012; Scussel et al. 2012b; Kreibich, 2017b).
CONCLUSIONS
Through the use of stereo and scanning electron microscopy we determined the main morpho-histological structures and cell characteristics related to susceptibility to fungi spoilage in andiroba seeds. The retention of placental residues, the uneven seed surface, the shell locule with attached channel and porous rich cells with plasmodesmata, and the presence of cracks and microcracks that favor insect and rodent attacks, may facilitate the infiltration of moisture and the attachment and proliferation of fungal conidia, leading to seed deterioration. Collection devices that prevent the seeds from falling to the ground, ventilation during transportation, and immediate cleaning, drying and dispatching are recommended to minimize the risk of spoilage of the seeds in the hot and humid conditions of the collection sites. This is the first study showing the different structures of the andiroba seed cells by SM and SEM.
ACKNOWLEDGMENTS
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship provided to BAS, and the Laboratory of Electron Microscopy Centre (LCME) at Uiversidade Federal de Santa Catarina for sharing the equipment and technical support.
REFERENCES
- Almeida, S.S.; Sousa, D.G.; Vale, N.C. 2009. História natural, ecologia e técnicas de manejo em castanhais nativos do sul do Amapá. In: Kanzaki, L.I.B. (Org.). Desenvolvimento Sustentável em Áreas de Extrativismo da Castanha-do-Brasil no Sul do Amapá Banco da Amazônia, Belém, 237p.
-
Alves, R.V. 2010. Estudo de caso da comercialização dos produtos florestais não madereiros (PFNM) como subsídio para a restauração florestal. Master’s dissertation, Universidade Federal de Viçosa, Brazil, 211p. (http://locus.ufv.br/handle/123456789/3040).
» http://locus.ufv.br/handle/123456789/3040 - Aragão, D.D.S.; Lunz, A.M.P.; Oliveira, L.C.D.; Raposo, A.; Fermino Junior, P.C.P. 2014. Efeito do sombreamento na anatomia foliar de plantas jovens de andiroba (Carapa guianensis Aubl.).Revista Árvore38: 631-639.
- Cabral, A.L.A.; Moras Filho, L.O.; Borges, L.A.C. 2013. Uso do fogo na agricultura: legislação, impactos ambientais e realidade na Amazônia.Fórum Ambiental da Alta Paulista9: 159-172.
- Camargo, J.L.C.; Ferraz, I.D.K.; Mesquita, M.R.; Santos, B.A.; Brum, H.D. 2008. Guia de Propágulos e Plântulas da Amazônia, v. 1, Editora INPA, Manaus, 300p.
- Carvalho, N.M.; Nakagawa, J. 2000. Sementes: Ciência, Tecnologia e Produção, 4th ed., FUNEP, Jaboticabal, 588p.
- Crawley, M.J.; Gillman, M.P. 1989. Population dynamics of cinnabar moth and ragwort in grassland.Journal of Animal Ecology 58: 1035-1050.
- Dias, K.K.B.; Cardoso, A.L.; da Costa, A.A.F.; Passos, M.F.; da Costa, C.E.F.; da Rocha Filho, G.N.; et al. 2023. Biological activities from andiroba (Carapa guianensis Aublet.) and its biotechnological applications: A systematic review.Arabian Journal of Chemistry16: 104629.
- Ferrari, M.; Oliveira, M.S.C.; Nakano, A.K.; Rocha-Filho, P.A. 2007. Determinação do fator de proteção solar (FPS) in vitro e in vivo de emulsões com óleo de andiroba (Carapa guianensis). Revista Brasileira de Farmacognosia 17: 626-630.
- Ferraz, I.D.K.; Camargo, J.L.C.; Sampaio, P.D.T.B. 2002. Sementes e plântulas de andiroba (Carapa guianensis Aubl. e Carapa procera DC): aspectos botânicos, ecológicos e tecnológicos.Acta Amazonica32: 647-647.
- Firmino, A.V.; Vidaurre, G.B.; Oliveira, J.T.D.S.; Guedes, M.; Almeida, M.N.F.D.; Silva, J.G.M.D.; Zanuncio, J.C. 2019. Wood properties of Carapa guianensis from floodplain and upland forests in Eastern Amazonia, Brazil.Scientific Reports9: 10641.
- Fisch, S.T.V. 1990. Comparações morfológicas e fisiológicas durante os processos de germinação de sementes e crescimento de plântulas de Carapa guianensis Aubl. e Carapa procera D.C. Master’s dissertation, Instituto Nacional de Pesquisas da Amazônia (INPA), Brazil, 102p.
- Guariguata, M.R.; Adame, J.J.R.; Finegan, B. 2000. Seed removal and fate in two selectively logged lowland forests with constrasting protection levels.Conservation Biology14: 1046-1054.
- Jordão, A.L.; Silva, R.A. 2006.Guia de Pragas Agrícolas Para o Manejo Integrado no Estado do Amapá Holos Editora, Rio de Janeiro, 150p.
- Kenfack, D. 2011. A synoptic revision of Carapa (Meliaceae).Harvard Papers in Botany16: 171-231.
- Klauck, V.; Pazinato, R.; Radavelli, W.M.; Volpato, A.; Stefani, L.M.; Silva, A.S. 2015. In vitro repellent effect of tea tree (Melaleuca alternifolia) and andiroba (Carapa guianensis) oils on Haematobia irritans and Chrysomya megacephala flies. Tropical Biomedicine 32: 33-39.
- Kreibich, H.H.; Moecke, E.O.E.; Scussel, V.M. 2017a. Stereo and scanning electron microscopy of whole post-fermentation dry cbcoa (Theobroma cacao L.): Part one - Healthy beans. v.7. In: Méndez-Vilas, A. (Ed.). Microscopy and Imaging Science: Practical Approaches to Applied Research and Education. Formatex Research Center, Badajoz, p.337-347.
- Kreibich, H.H.; Moecke, E.O.E.; Scussel, V.M. 2017b. Stereo and scanning electron microscopy of cocoa beans (Theobroma cacao L.): Part two - Fungi spoilage susceptibility. v.7. In: Méndez-Vilas, A. (Ed.). Microscopy and Imaging Science: Practical Approaches to Applied Research and Education. Formatex Research Center, Badajoz , p.329-336.
- Lorenzi, H. 2002. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 4th ed. Instituto Plantarium, Nova Odessa, 384p.
- Manfio, D.; Beirao, L.H.; Damian, C.; Savi, G.D.; Scussel, V.M. 2012. Brazil nut (Bertholettia excels H.B.K.) brown skin characterization - a waste product generated from shelled dry nut factories of Amazon region. Agricultural Sciences Research Journal 2: 253-260.
-
Matos, R.S.; Ţălu, Ş.; Mota, G.V.; Pinto, E.P.; Pires, M.A.; Abraçado, L.G.; Ferreira, N.S. 2021. Correlating structure and morphology of andiroba leaf (Carapa guianensis Aubl.) by microscopy and fractal theory analyses.Applied Sciences 11: 5848. doi.org/10.3390/app11135848.
» https://doi.org/10.3390/app11135848 - Maule, A.J. 2008. Plasmodesmata: structure, function and biogenesis.Current Opinion in Plant Biology11: 680-686.
- McHargue, L.A.; Hartshorn, G.S. 1983. Seed and seedling ecology of Carapa guianensis.Turrialba 33: 399-404.
- Mendonça, A.P.; Ferraz, I.D.K. 2007. Óleo de andiroba: processo tradicional da extração, uso e aspectos sociais no estado do Amazonas, Brasil.Acta Amazonica37: 353-364.
- Moraes, E.A.; Bianchin, I.; Silva, K.F.D.; Catto, J.B.; Honer, M.R.; Paiva, F. 2010. Anthelmintic resistance of gastrointestinal nematodes in sheep, Mato Grosso do Sul, Brazil. Pesquisa Veterinária Brasileira 30: 229-236.
- Moura, C.V.R.; Silva, B.C.; Castro, A.G.; Moura, E.M.; Veloso, M.D.C.; Sittolin, I.M.; Araujo, E.C.E. 2019. Caracterização físico-química de óleos vegetais de oleaginosas adaptáveis ao Nordeste Brasileiro com potenciais para produção de biodiesel. Revista Virtual Quimica 11: 573-595.
-
Pereira, T.B.; Silva, L.F.R.; Amorim, R.C.; Melo, M.R.; Souza, R.C.Z.; Eberlin, M.N.; Pohlit, A.M. 2014. In vitro and in vivo anti-malarial activity of limonoids isolated from the residual seed biomass from Carapa guianensis (andiroba) oil production. Malaria Journal 13: 317. doi.org/10.1186/1475-2875-13-317
» https://doi.org/10.1186/1475-2875-13-317 -
Pinto, A.A. 2007. Avaliação de danos causados por insetos em sementes de andiroba (Carapa guianensis Aubl.) e andirobinha (C. procera) (Meliaceae) na reserva florestal Ducke em Manaus, AM. Brasil. Master’s dissertation, Instituto Nacional de Pesquisa da Amazônia, Brazil, 60p. (https://repositorio.inpa.gov.br/handle/1/12426).
» https://repositorio.inpa.gov.br/handle/1/12426 - Plowden, C. 2004. The ecology and harvest of andiroba seeds for oil production in the Brazilian Amazon.Conservation and Society 2: 251-272.
- Santos, R.S.; Pellicciotti, A.S. 2016. Ocorrência de Hypsipyla ferrealis Hampson (Lepidoptera: Pyralidae) em andiroba no estado do Acre.Ciência Florestal26: 995-998.
- Scussel, V.M.; Manfio, D.; Savi, G.D.; Moecke, E.H. 2014a. Stereoscopy and scanning electron microscopy of Brazil nut (Bertholletia excelsa HBK) shell, brown skin, and edible part: Part one - Healthy nut.Journal of Food Science79: H1443-H1453.
- Scussel, V.M.; Manfio, D.; Savi, G.D.; Moecke, E.H.S. 2014b. Stereo and scanning electron microscopy of in-shell Brazil nut (Bertholletia excelsa H.B.K.) Part two -Surface sound nut fungi spoilage susceptibility. Journal Food Science 79: H2392 - H2403.
- Segovia, J.F.O.; Oliveira, V.L.; Gonçalves, M.C.A.; Resck, I.S.; Silva, C.A.M.; Silveira, D.; Kanzaki, L.I.B. 2011. Botanical characterisation, geographical distribution and phytochemistry analysis of Manilkara huberi (Ducke) Standl autochtonous in Amapá State, Brazil.Proceedings of the National Academy of Sciences of Belarius, Biological Series 2:35-40.
- Silva, B.A.; Silva, N.C.; Runtzel, C.L.; Aquino, C.M.; Scussel, V.M. 2019. Effect of Andiroba (Carapa guianensis Aubl.) oil for fungi control in maize (Zea Mays L.) grains. IOSR Journal of Agriculture and Veterinary Science 12: 26-36.
- Silva, B.A.; Scussel, V.M. 2020. Characteristics and effects of the Amazonian andiroba (Carapa guianensis Aubl.) oil against living organisms - A review. Journal of Biotechnology and Biochemistry 6: 31-47.
- Sousa, S.F.; Paes, J.B.; Arantes, M.D.C.; Lopez, Y.M.; Brocco, V.F. 2018. Análise física e avaliação do efeito antifúngico dos óleos de andiroba, copaíba e pinhão-manso. Floresta 48: 153-162.
- Tsukamoto, Y.; Oya, H.; Kikuchi, T.; Yamada, T.; Tanaka, R. 2019. Guianofruits C-I from fruit oil of andiroba (Carapa guianensis Aubl.) Meliaceae.Tetrahedron75: 1149-1156.
Data availability
The data that support the findings of this study can be made available, upon reasonable request, from the corresponding author, Bruna Aparecida da Silva.
Publication Dates
-
Publication in this collection
17 Mar 2025 -
Date of issue
2025
History
-
Received
25 May 2023 -
Accepted
09 Oct 2024

 Anatomical study of the seed of andiroba (Carapa guianensis) through stereo and scanning electron microscopy
Anatomical study of the seed of andiroba (Carapa guianensis) through stereo and scanning electron microscopy